Controlled Release Introduction and Background 1 Topics n

Controlled Release Introduction and Background 1

Topics n n Definitions Classifications of CR Systems q n n n Rate control, physical form Design considerations Routes of administration Review of mass transfer 2

Definition of Controlled Release n A system that: q q q Delivers an agent at a controlled rate for an extended time Might localize drug action by spatial placement near where it is needed Might target drug action by using techniques to deliver drug to a particular cell type 3

Controlled Release Agents n In nature (? ) q q q Oxygenation of blood Transport of nutrients and waste through cell membranes Transport and evaporation of water (sweat) to control body temperature n Engineered systems (? ) q q q Drugs Biocides Fragrances 4

Controlled Release vs. Sustained Release n Controlled drug delivery q q Well-characterized and reproducible dosage form Controls entry to the body according to the specifications of the required drug delivery profile n n rate and duration of delivery are designed to achieve desired concentration Sustained Release q q Release of drug is extended in time Rate and duration are not designed to achieve a particular profile. 5
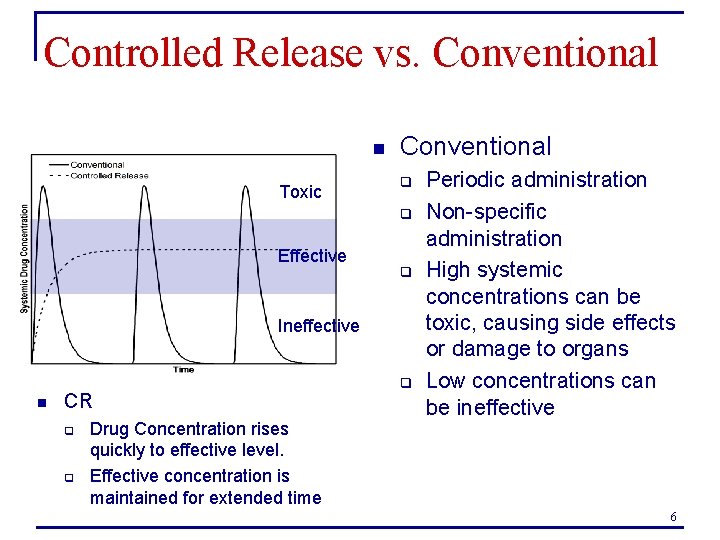
Controlled Release vs. Conventional n Toxic Conventional q q Effective q Ineffective n CR q q Drug Concentration rises quickly to effective level. Effective concentration is maintained for extended time q Periodic administration Non-specific administration High systemic concentrations can be toxic, causing side effects or damage to organs Low concentrations can be ineffective 6

Disadvantages of Conventional Delivery (Brainstorm) n n n Inconvenient Difficult to monitor Careful calculation necessary to prevent overdosing Large amounts of drug can be “lost” when they don’t get to the target organ Drug goes to non-target cells and can cause damage Expensive (using more drug than necessary) 7

Advantages of Controlled Release (Brainstorm) n n Reproducible rate, prolonged delivery Less frequent administration q q n n Better patient compliance Increased convenience Reduced side effects because effective C is maintained Targeting can eliminate damage to non-target organs Less drug used Re-patenting without new drug development 8

Challenges to Controlled Release n n Cost of formulation – preparation and processing Fate of controlled release system if not biodegradable Biocompatibility Fate of polymer additives, e. g. , plasticizers, stabilizers, antioxidants, fillers 9
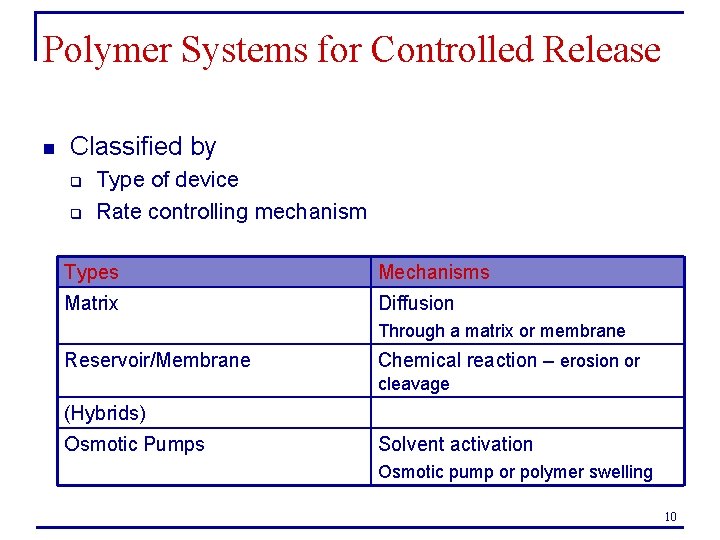
Polymer Systems for Controlled Release n Classified by q q Type of device Rate controlling mechanism Types Mechanisms Matrix Diffusion Through a matrix or membrane Reservoir/Membrane Chemical reaction – erosion or cleavage (Hybrids) Osmotic Pumps Solvent activation Osmotic pump or polymer swelling 10
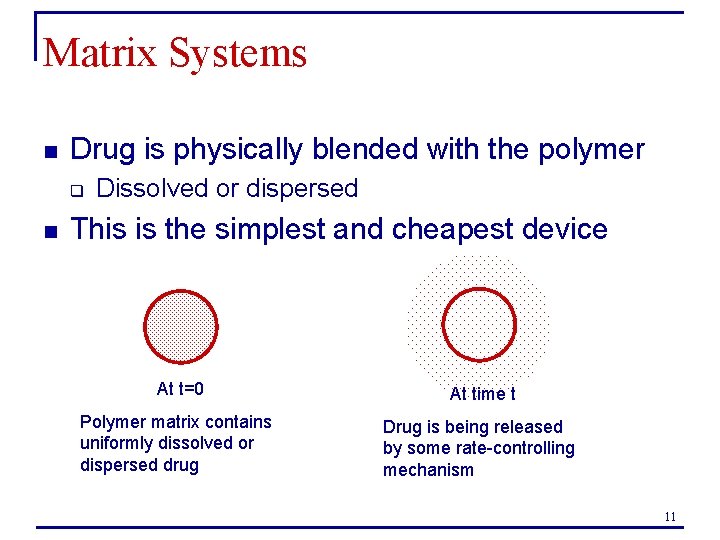
Matrix Systems n Drug is physically blended with the polymer q n Dissolved or dispersed This is the simplest and cheapest device At t=0 At time t Polymer matrix contains uniformly dissolved or dispersed drug Drug is being released by some rate-controlling mechanism 11
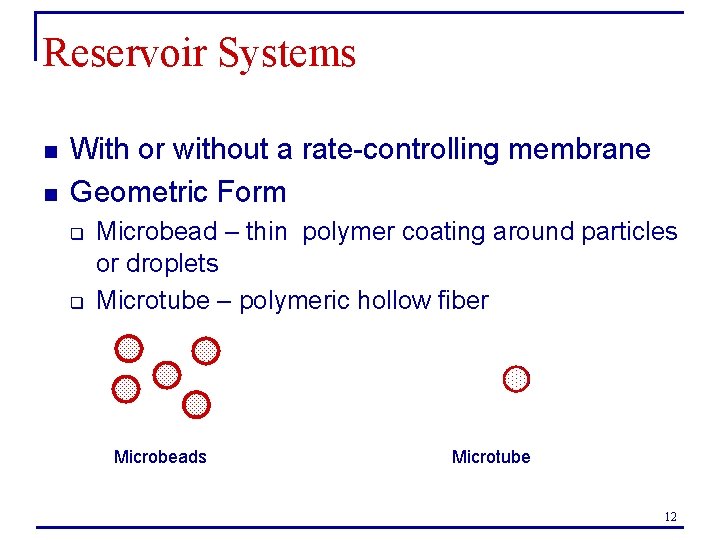
Reservoir Systems n n With or without a rate-controlling membrane Geometric Form q q Microbead – thin polymer coating around particles or droplets Microtube – polymeric hollow fiber Microbeads Microtube 12
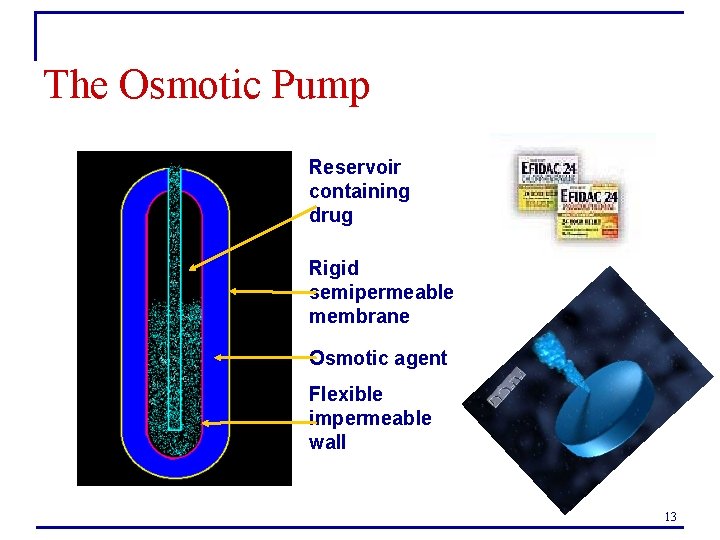
The Osmotic Pump Reservoir containing drug Rigid semipermeable membrane Osmotic agent Flexible impermeable wall 13
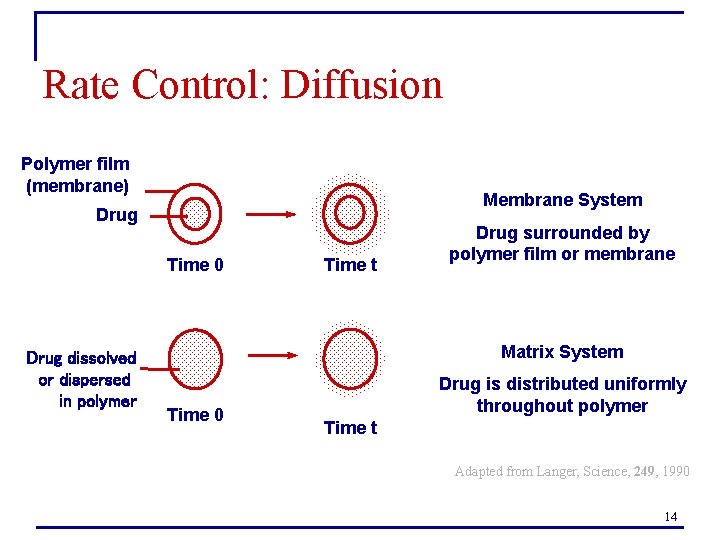
Rate Control: Diffusion Polymer film (membrane) Membrane System Drug Time 0 Drug dissolved or dispersed in polymer Time t Drug surrounded by polymer film or membrane Matrix System Time 0 Drug is distributed uniformly throughout polymer Time t Adapted from Langer, Science, 249, 1990 14

Diffusion Systems Contac® 12 Hour Cold Capsules Ocusert® (Pilocarpine for Glaucoma) Nocoderm® Patch 15
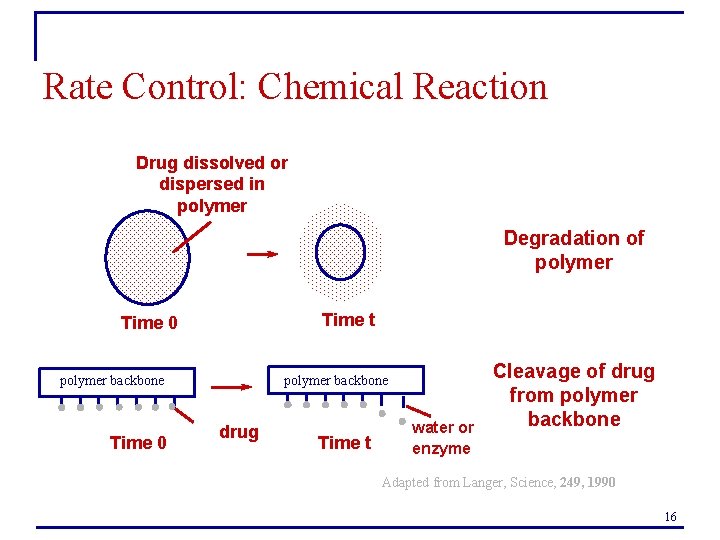
Rate Control: Chemical Reaction Drug dissolved or dispersed in polymer Degradation of polymer Time t Time 0 polymer backbone Time 0 drug Time t water or enzyme Cleavage of drug from polymer backbone Adapted from Langer, Science, 249, 1990 16

Biodegradable Systems n n Implants for release of anticancer drugs Lupron Depot® q q q n Injectable microspheres Once per month injecton Prostrate cancer, fertility treatment, early puberty Malaria vaccine 17

Rate Control: Solvent Activation Swollen Polymer from which drug has been released Drug dissolved in polymer Swelling allows drug to migrate more easily Time 0 Time t Drug dispersed in polymer Pores permit release Time 0 Time t Osmotic pressure causes water to penetrate, forming pores and releasing drug Adapted from Langer, Science, 249, 1990 18

Design Considerations n Basic components q q n Active agent Polymer design considerations (? ) q Physical properties n n q q q Glass transition temperature Diffusion characteristics Compatibility with active Stability – must not decompose in storage Biocompatibility of polymer and degradation products Ease of formulation and fabrication Mechanical properties are stable when drug is added Cost 19

Design considerations n Agent q Physicochemical properties n n n Stability Solubility Partitioning Charge Protein binding propensity 20
Design Considerations n n Route of delivery Target sites q q n Desired site for efficacy Sites to avoid to minimize side effects Type of therapy q Acute or chronic – rate and duration n n e. g. , 1 yr contraceptive implant vs. antibiotic for acute infection Patient condition q q Cognative ability and memory Physical condition – ambulatory, bedridden, etc. 21

Routes of Administration for CR n Parenteral – outside GI tract q Usually refers to injectables n n Subcutaneous Intramuscular Intraperitoneal Intravenous n Advantages q n Bypasses some routes of metabolic clearance Disadvantages (? ) q q Painful Inconvenient 22
Routes of Administration n Oral q Most common route n n n q Easy to formulate and manufacture Patient compliance is generally good Inexpensive dosage form Tricky due to environment of GI tract n n n p. H degradation Enzymatic degradation Intestinal motility – affects residence time q n Single patient and patient-to-patient variations Absorption limitations in stomach 23
Routes of Administration n Buccal/ Sublingual q q q n n Thin mucous membrane Rich blood supply Mild p. H ~6. 0 Rectal q q Nasal q q q Easy administration Rapid absorption Bypasses certain clearance routes q n No p. H or enzymatic degradation as in oral (+) More effective than buccal or sublingual for some drugs (+) Limited absorption (-) Pulmonary q Large S. A. for absorption 24

Routes of Administration n Transdermal q q q n Accessible organ, large surface area Avoid first pass metabolism Avoid GI incompatibility of drugs Good patient compliance Transport across skin can be a challenge Ocular q q q Localized delivery for eye disorders Good absorption for many drugs Loss of drug in tears 25
- Slides: 25