CONTROL VOLUMES I am teaching Engineering Thermodynamics using









- Slides: 45

CONTROL VOLUMES I am teaching Engineering Thermodynamics using the textbook by Cengel and Boles. This set of slides overlap with Chapters 5 and 7. Some figures in the slides are taken from that book, and some others are found online. Similar figures can be found in many places. I went through these slides in two lectures, each 90 minutes. Zhigang Suo

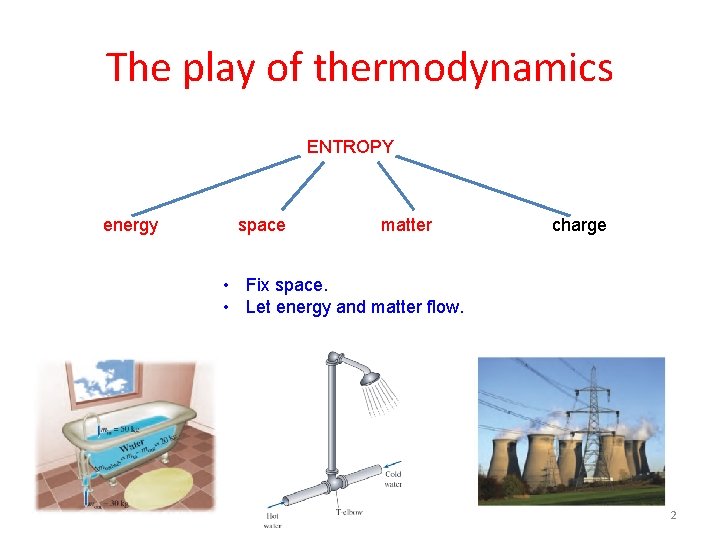
The play of thermodynamics ENTROPY energy space matter charge • Fix space. • Let energy and matter flow. 2

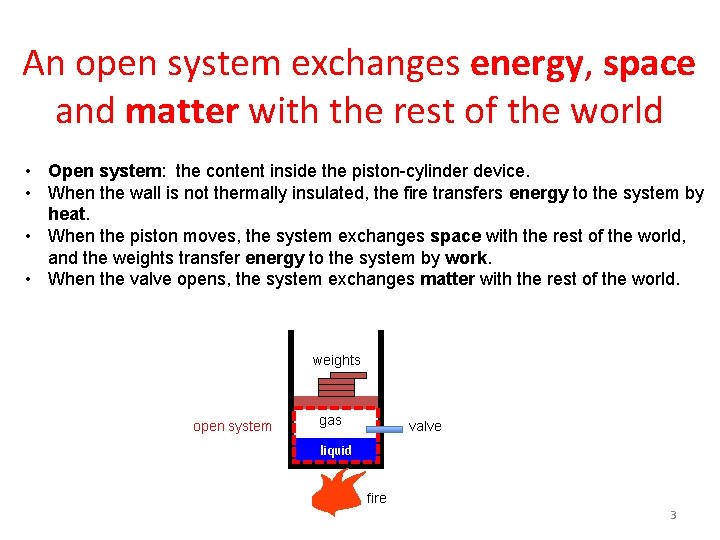
An open system exchanges energy, space and matter with the rest of the world • Open system: the content inside the piston-cylinder device. • When the wall is not thermally insulated, the fire transfers energy to the system by heat. • When the piston moves, the system exchanges space with the rest of the world, and the weights transfer energy to the system by work. • When the valve opens, the system exchanges matter with the rest of the world. weights open system gas valve liquid fire 3

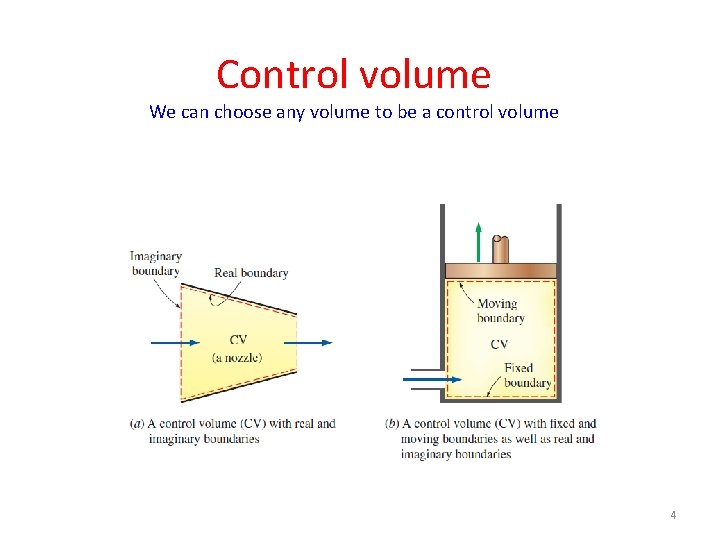
Control volume We can choose any volume to be a control volume 4

Plan • • • Conservation of mass Conservation of energy Generation of entropy Steady-flow devices Isentropic efficiency of steady-flow devices Reversible work of steady-flow devices 5

Isolated system When confused, isolate. Isolated system IS Isolated system conserves mass over time: 6

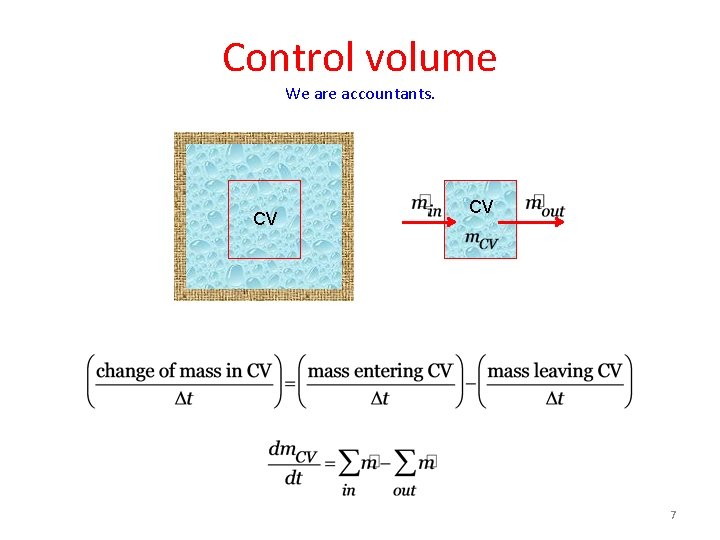
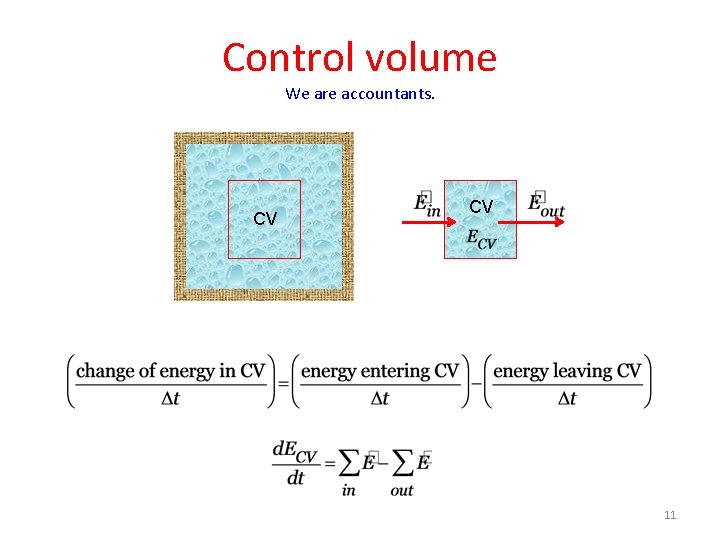
Control volume We are accountants. CV CV 7

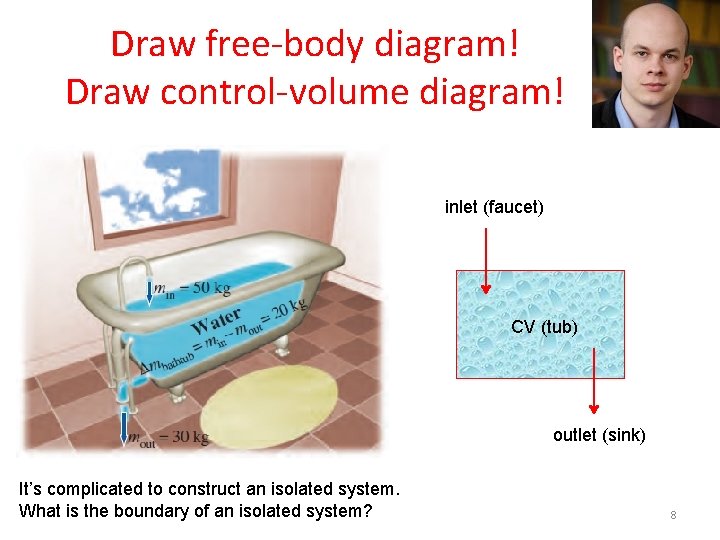
Draw free-body diagram! Draw control-volume diagram! inlet (faucet) CV (tub) outlet (sink) It’s complicated to construct an isolated system. What is the boundary of an isolated system? 8

Plan • • • Conservation of mass Conservation of energy Generation of entropy Steady-flow devices Isentropic efficiency of steady-flow devices Reversible work of steady-flow devices 9

Isolated system When confused, isolate. Isolated system IS Isolated system conserves energy over time: 10

Control volume We are accountants. CV CV 11

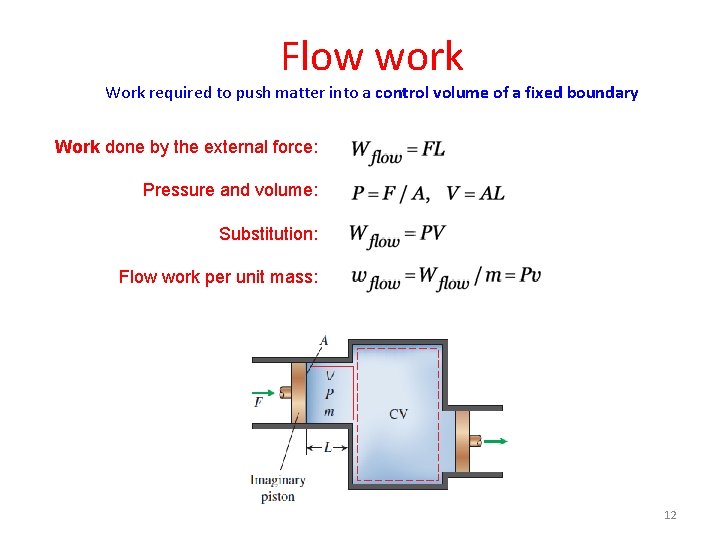
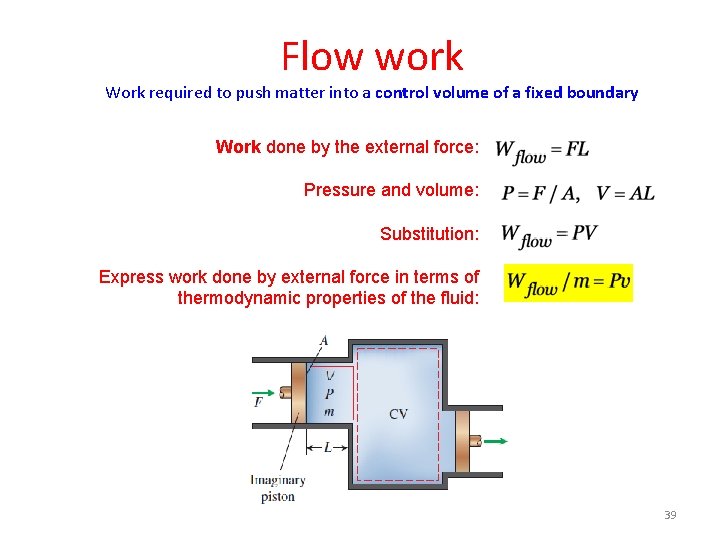
Flow work Work required to push matter into a control volume of a fixed boundary Work done by the external force: Pressure and volume: Substitution: Flow work per unit mass: 12

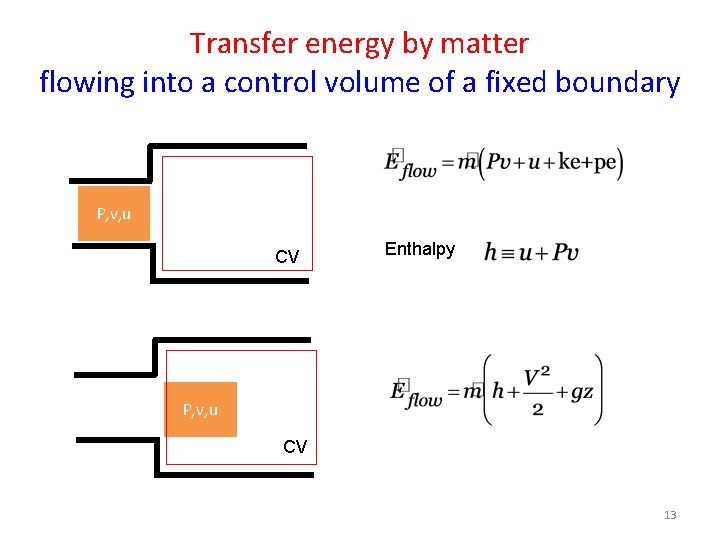
Transfer energy by matter flowing into a control volume of a fixed boundary P, v, u CV Enthalpy P, v, u CV 13

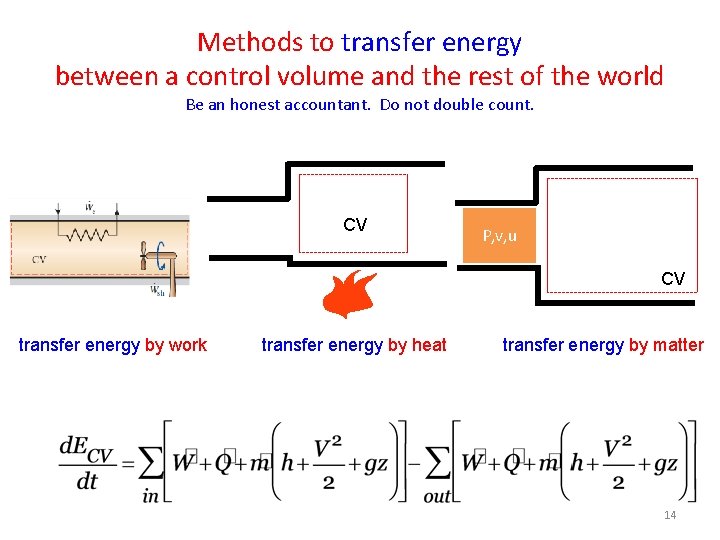
Methods to transfer energy between a control volume and the rest of the world Be an honest accountant. Do not double count. CV P, v, u CV transfer energy by work transfer energy by heat transfer energy by matter 14

Plan • • • Conservation of mass Conservation of energy Generation of entropy Steady-flow devices Isentropic efficiency of steady-flow devices Reversible work of steady-flow devices 15

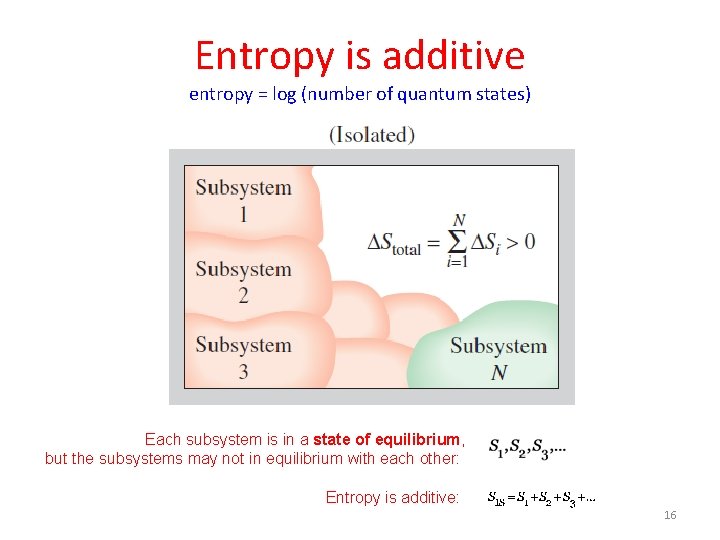
Entropy is additive entropy = log (number of quantum states) Each subsystem is in a state of equilibrium, but the subsystems may not in equilibrium with each other: Entropy is additive: 16

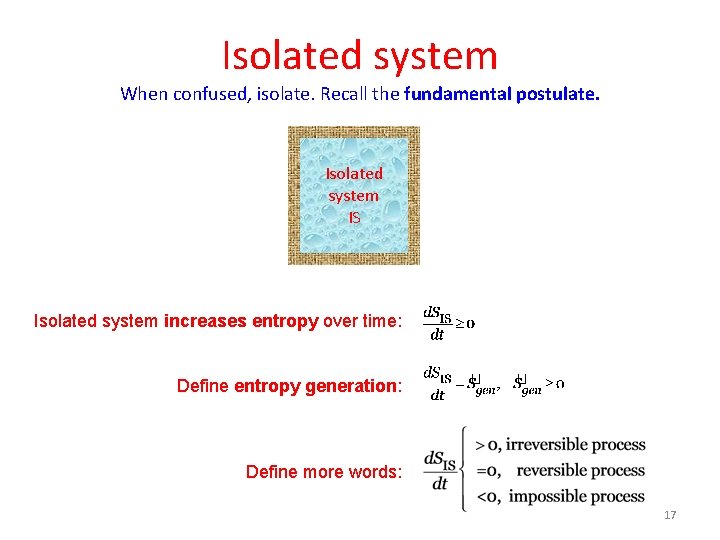
Isolated system When confused, isolate. Recall the fundamental postulate. Isolated system IS Isolated system increases entropy over time: Define entropy generation: Define more words: 17

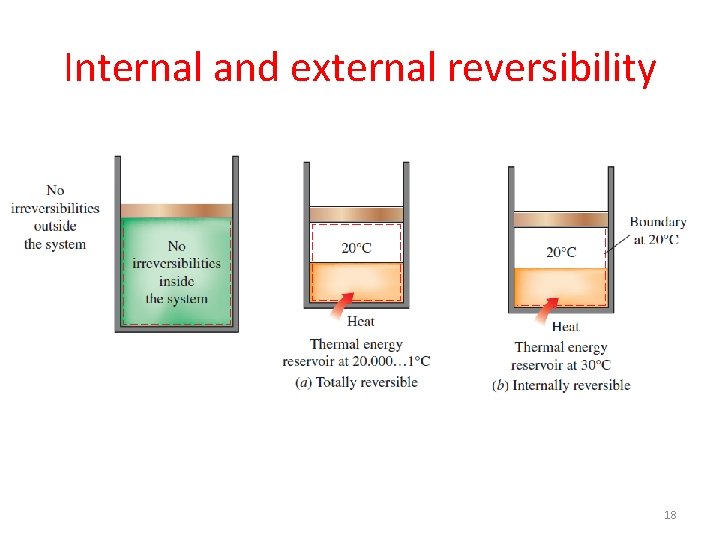
Internal and external reversibility 18

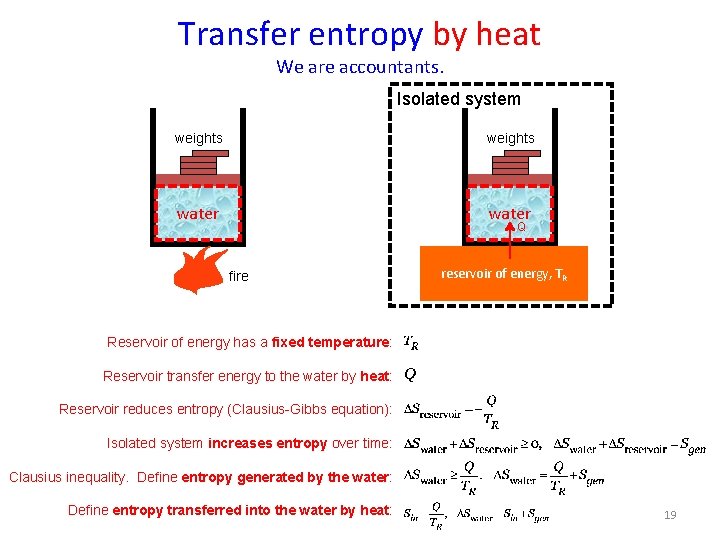
Transfer entropy by heat We are accountants. Isolated system weights water Q fire reservoir of energy, TR Reservoir of energy has a fixed temperature: Reservoir transfer energy to the water by heat: Reservoir reduces entropy (Clausius-Gibbs equation): Isolated system increases entropy over time: Clausius inequality. Define entropy generated by the water: Define entropy transferred into the water by heat: 19

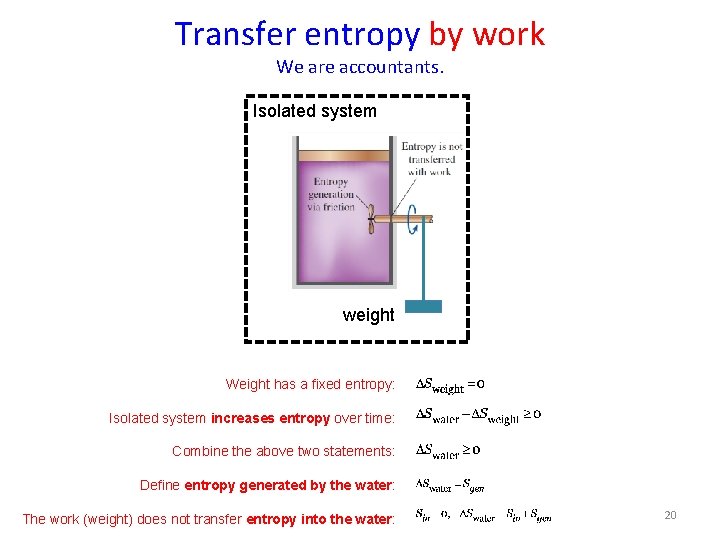
Transfer entropy by work We are accountants. Isolated system weight Weight has a fixed entropy: Isolated system increases entropy over time: Combine the above two statements: Define entropy generated by the water: The work (weight) does not transfer entropy into the water: 20

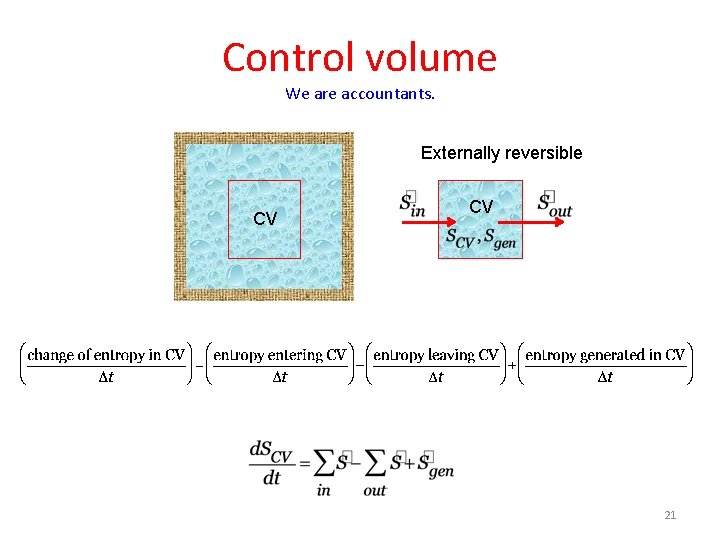
Control volume We are accountants. Externally reversible CV CV 21

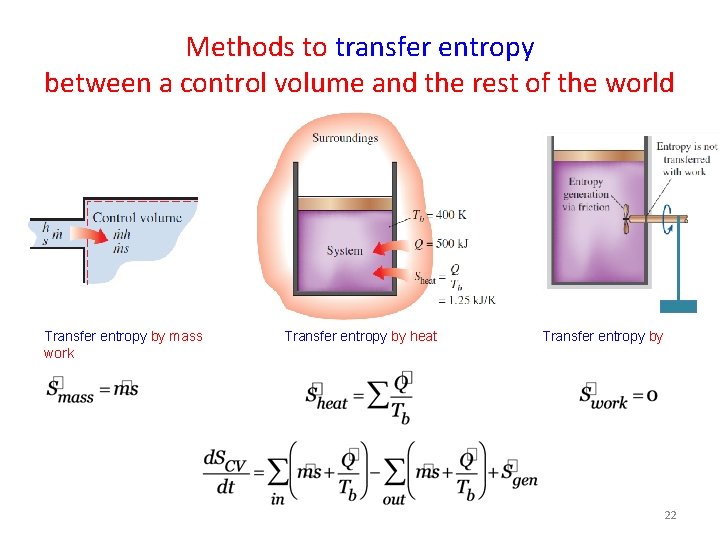
Methods to transfer entropy between a control volume and the rest of the world Transfer entropy by mass work Transfer entropy by heat Transfer entropy by 22

Plan • • • Conservation of mass Conservation of energy Generation of entropy Steady-flow devices Isentropic efficiency of steady-flow devices Reversible work of steady-flow devices 23

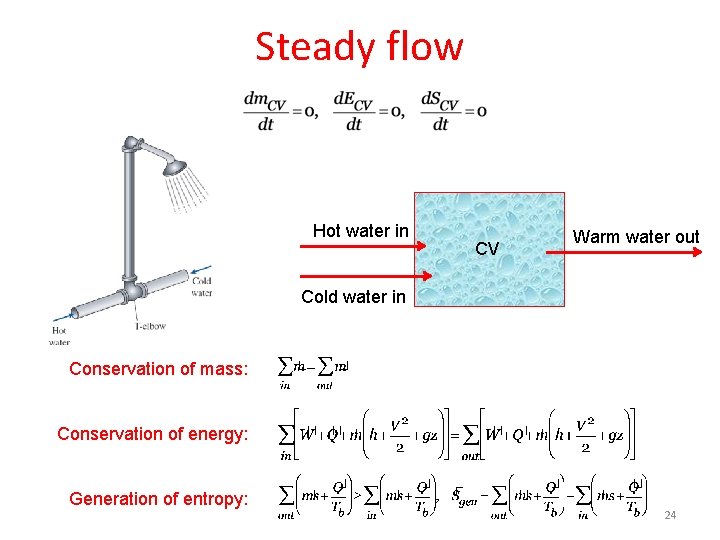
Steady flow Hot water in CV Warm water out Cold water in Conservation of mass: Conservation of energy: Generation of entropy: 24

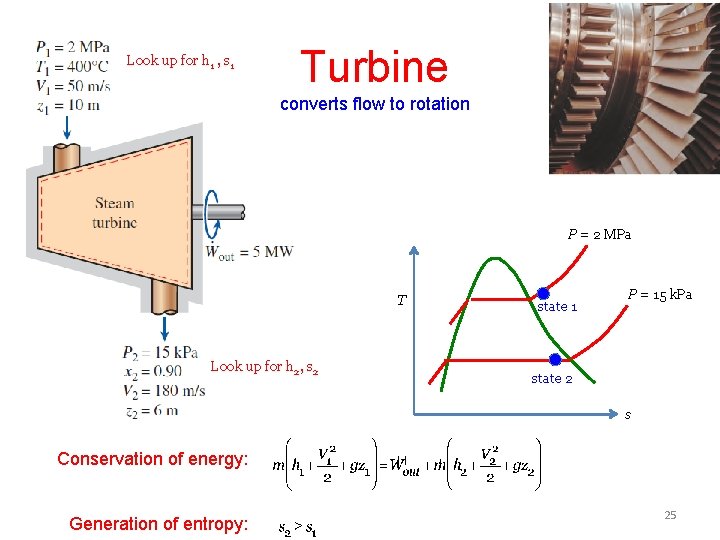
Look up for h 1 , s 1 Turbine converts flow to rotation P = 2 MPa T Look up for h 2, s 2 state 1 P = 15 k. Pa state 2 s Conservation of energy: Generation of entropy: 25

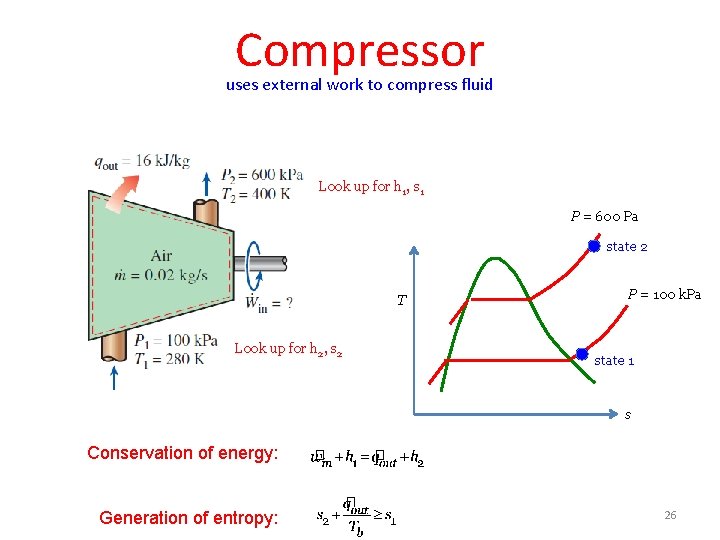
Compressor uses external work to compress fluid Look up for h 1, s 1 P = 600 Pa state 2 T Look up for h 2, s 2 P = 100 k. Pa state 1 s Conservation of energy: Generation of entropy: 26

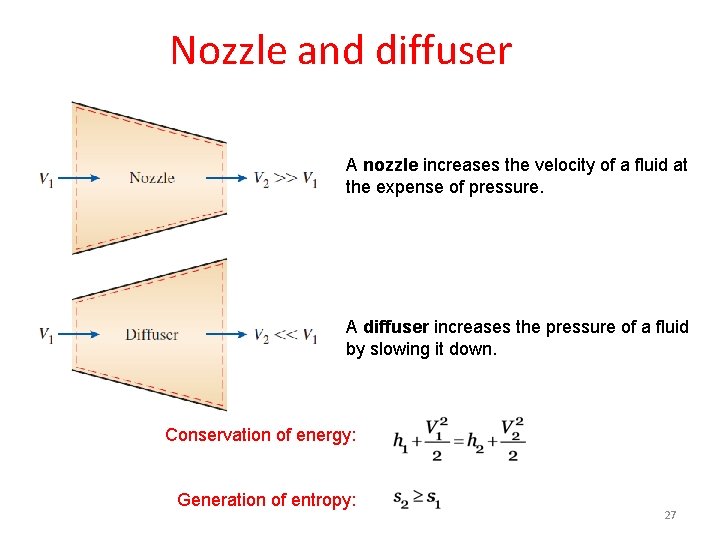
Nozzle and diffuser A nozzle increases the velocity of a fluid at the expense of pressure. A diffuser increases the pressure of a fluid by slowing it down. Conservation of energy: Generation of entropy: 27

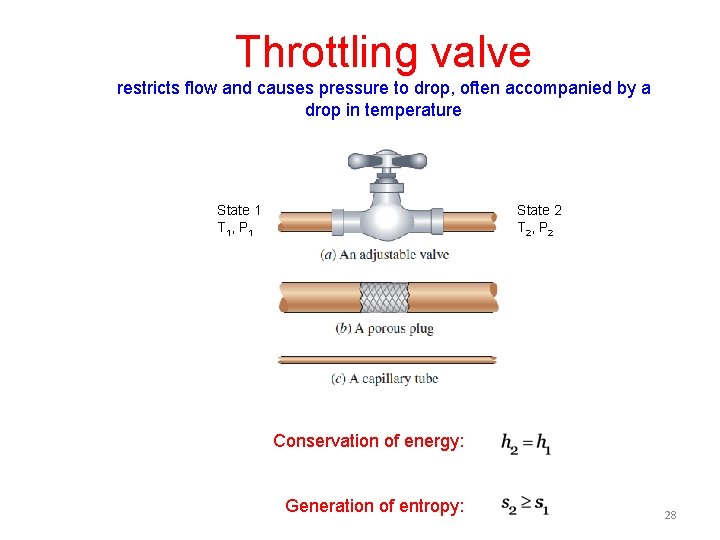
Throttling valve restricts flow and causes pressure to drop, often accompanied by a drop in temperature State 1 T 1, P 1 State 2 T 2, P 2 Conservation of energy: Generation of entropy: 28

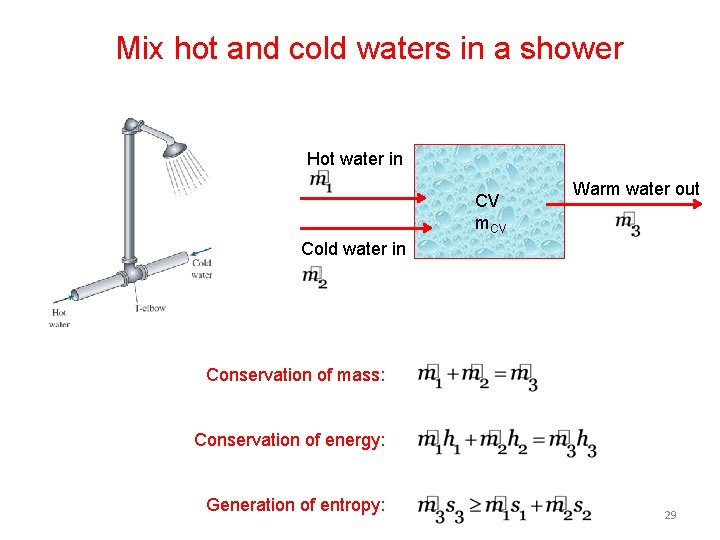
Mix hot and cold waters in a shower Hot water in CV m. CV Warm water out Cold water in Conservation of mass: Conservation of energy: Generation of entropy: 29

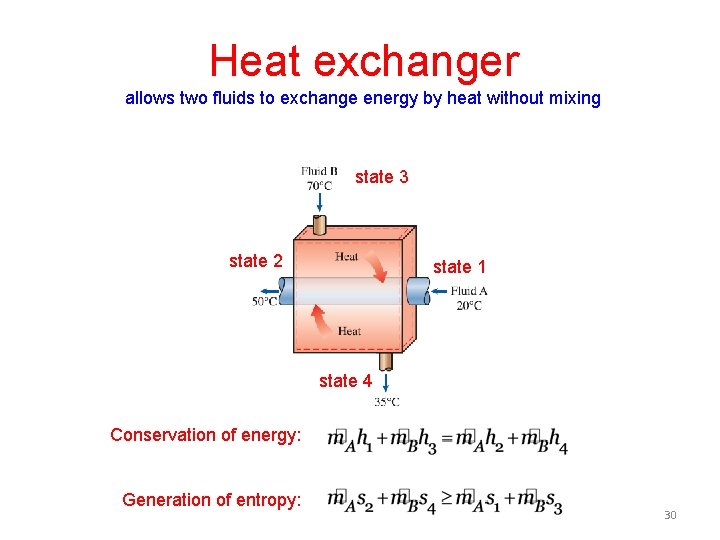
Heat exchanger allows two fluids to exchange energy by heat without mixing state 3 state 2 state 1 state 4 Conservation of energy: Generation of entropy: 30

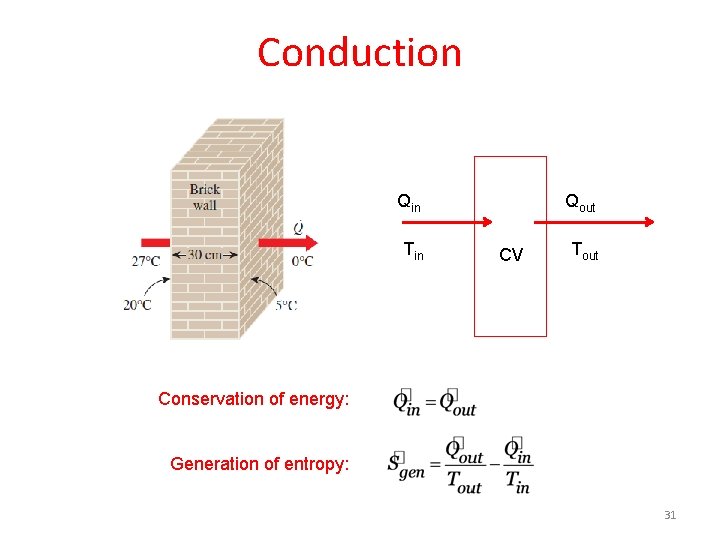
Conduction Qin Tin Qout CV Tout Conservation of energy: Generation of entropy: 31

Plan • • • Conservation of mass Conservation of energy Generation of entropy Steady-flow devices Isentropic efficiency of steady-flow devices Reversible work of steady-flow devices 32

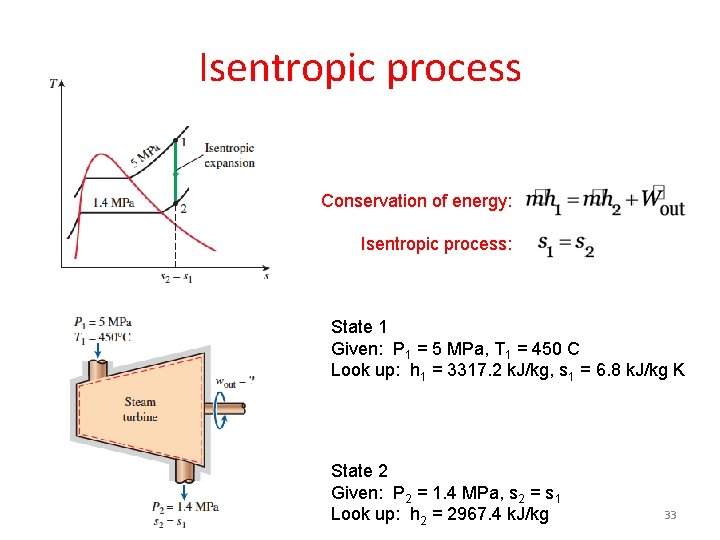
Isentropic process Conservation of energy: Isentropic process: State 1 Given: P 1 = 5 MPa, T 1 = 450 C Look up: h 1 = 3317. 2 k. J/kg, s 1 = 6. 8 k. J/kg K State 2 Given: P 2 = 1. 4 MPa, s 2 = s 1 Look up: h 2 = 2967. 4 k. J/kg 33

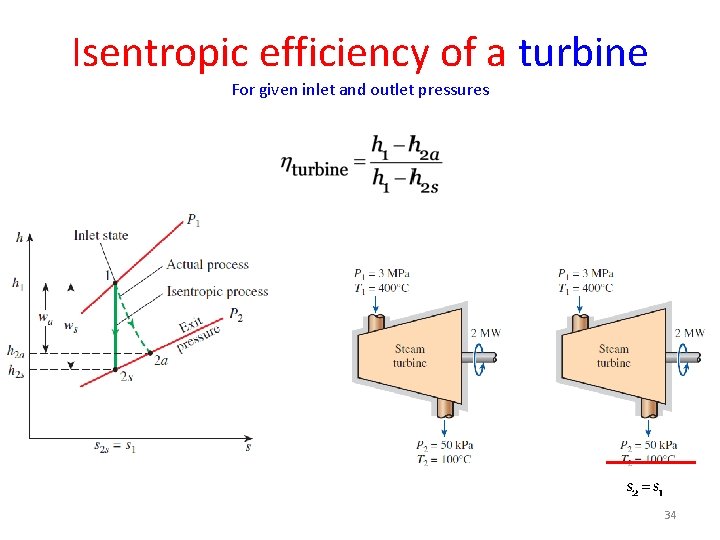
Isentropic efficiency of a turbine For given inlet and outlet pressures 34

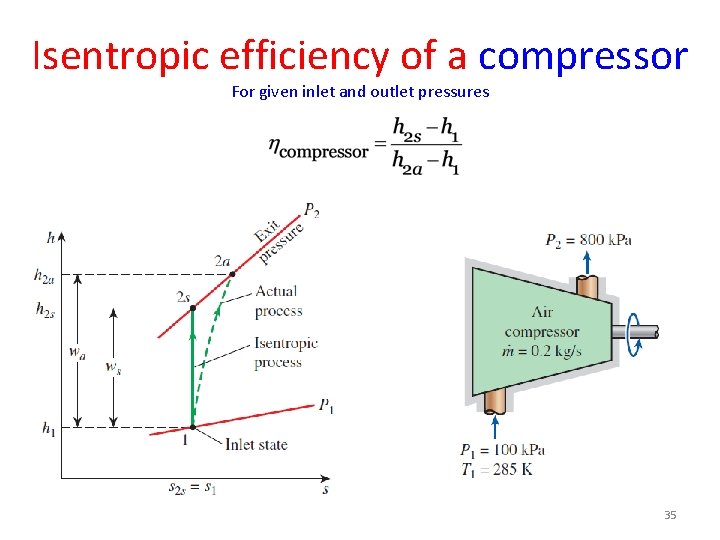
Isentropic efficiency of a compressor For given inlet and outlet pressures 35

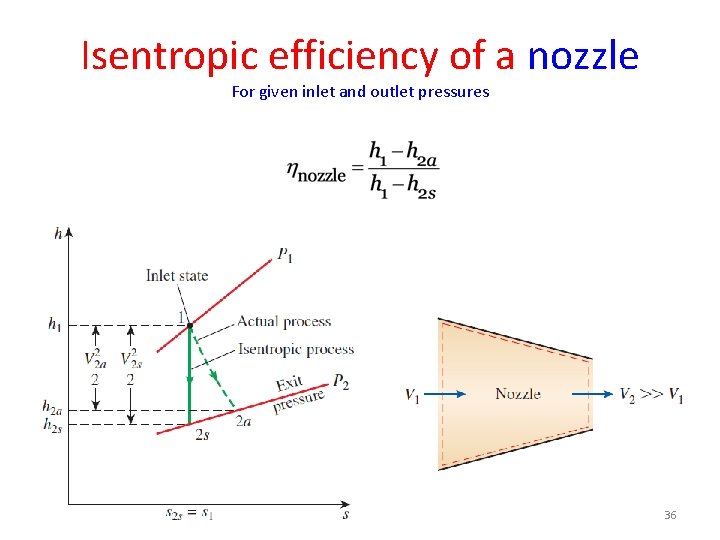
Isentropic efficiency of a nozzle For given inlet and outlet pressures 36

Plan • • • Conservation of mass Conservation of energy Generation of entropy Steady-flow devices Isentropic efficiency of steady-flow devices Reversible work of steady-flow devices 37

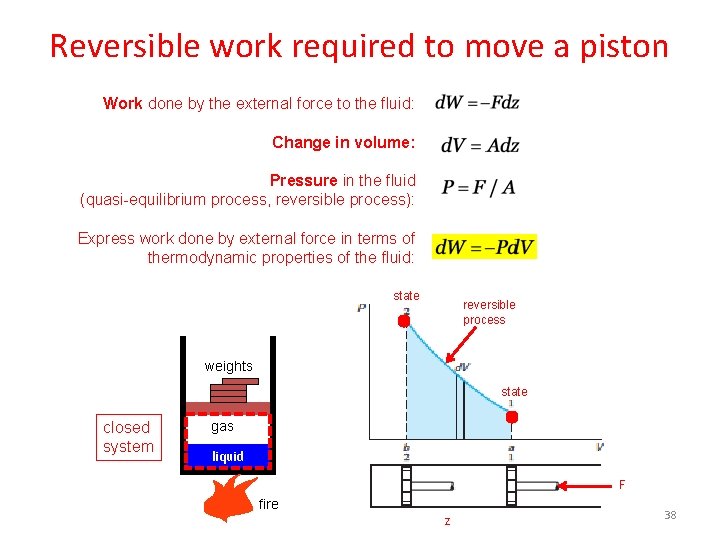
Reversible work required to move a piston Work done by the external force to the fluid: Change in volume: Pressure in the fluid (quasi-equilibrium process, reversible process): Express work done by external force in terms of thermodynamic properties of the fluid: state reversible process weights state closed system gas liquid F fire z 38

Flow work Work required to push matter into a control volume of a fixed boundary Work done by the external force: Pressure and volume: Substitution: Express work done by external force in terms of thermodynamic properties of the fluid: 39

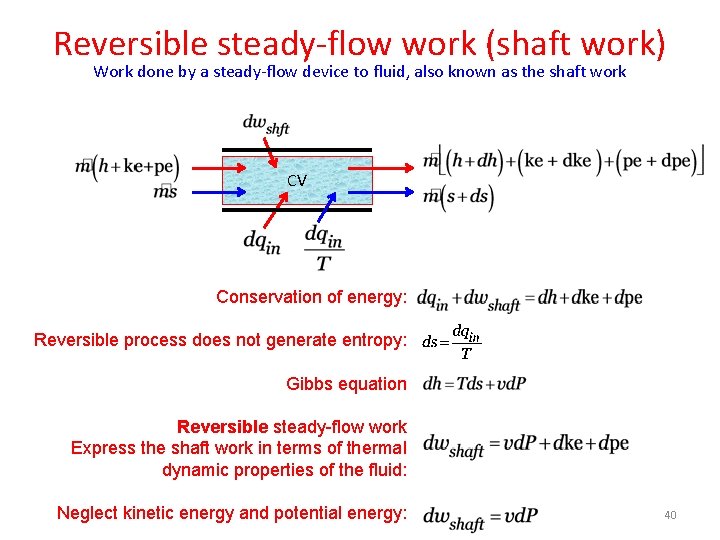
Reversible steady-flow work (shaft work) Work done by a steady-flow device to fluid, also known as the shaft work CV Conservation of energy: Reversible process does not generate entropy: Gibbs equation Reversible steady-flow work Express the shaft work in terms of thermal dynamic properties of the fluid: Neglect kinetic energy and potential energy: 40

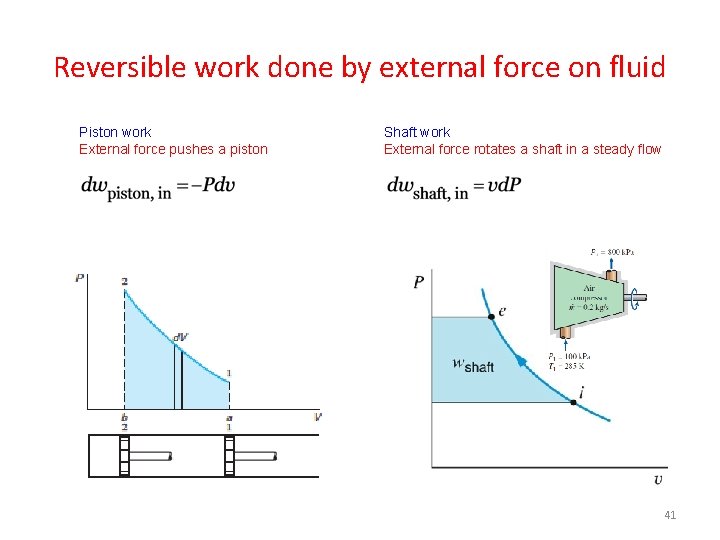
Reversible work done by external force on fluid Piston work External force pushes a piston Shaft work External force rotates a shaft in a steady flow 41

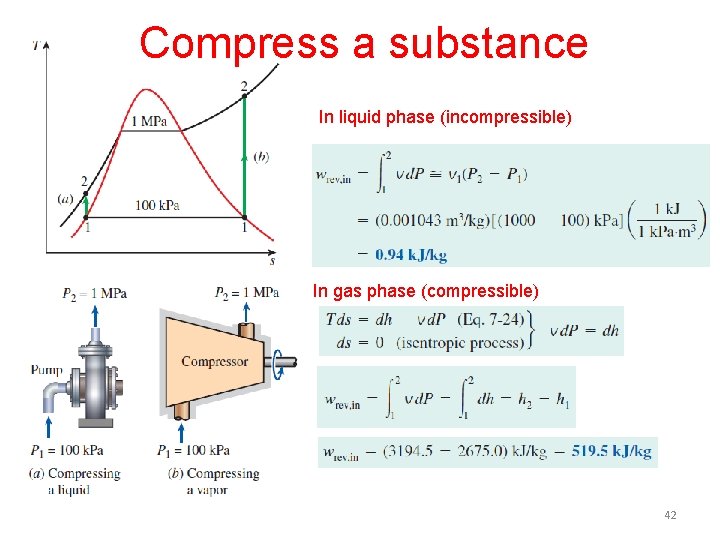
Compress a substance In liquid phase (incompressible) In gas phase (compressible) 42

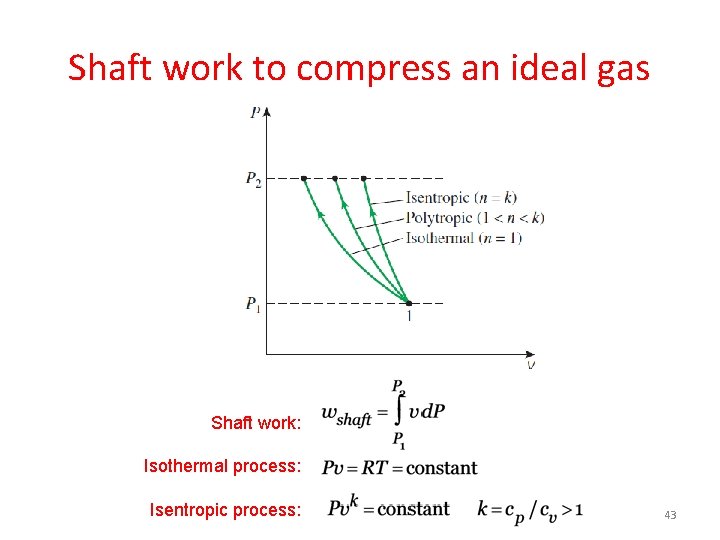
Shaft work to compress an ideal gas Shaft work: Isothermal process: Isentropic process: 43

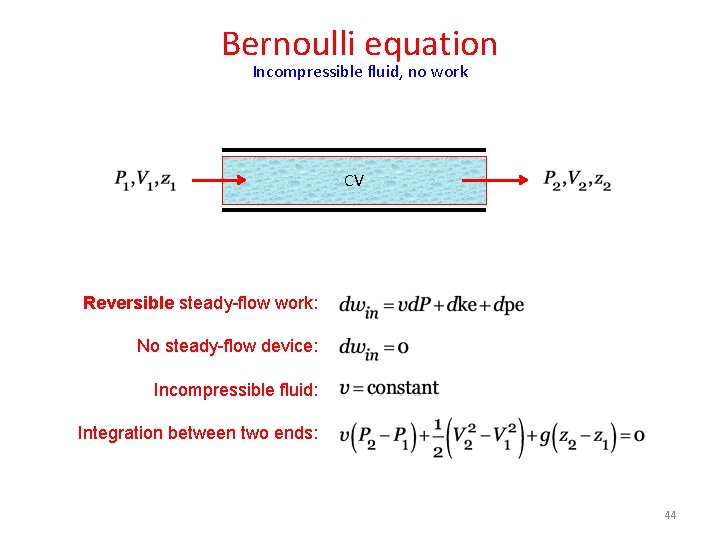
Bernoulli equation Incompressible fluid, no work CV Reversible steady-flow work: No steady-flow device: Incompressible fluid: Integration between two ends: 44

Summary • An isolated system conserves mass, conserves energy, but generates entropy. • Translate the above statement by labeling part of an isolated system as a control volume. • Steady-flow devices • Isentropic efficiency of steady-flow devices • Reversible work of steady-flow devices (shaft work) 45