Control Charts for CPV A Pharma Perspective Maneesha












- Slides: 41

Control Charts for CPV – A Pharma Perspective Maneesha Altekar, Principal Statistician, Astra. Zeneca MBSW Conference, May 22 -24, 2017, Muncie, IN

Overview • Why use Control Charts? • Type of Data vs Type of Control Chart • Calculating Limits • Responding to Signals - Western Electric Rules • Challenges 2

Overview • Why use Control Charts? • Type of Data vs Type of Control Chart • Calculating Limits • Responding to Signals - Western Electric Rules • Challenges 3

Background • 2011 FDA Guidance on Process Validation, EU Annex 15 • Demonstrate that the validated process continues to remain in a validated state – Continued Process verification (CPV) • Emphasis on the use of statistical methods 4

Control Charts • Statistical analysis methodology, used for CPV • Visually monitor process over time against established limits • Ensure that it is stable and in statistical control • • React to real changes in the process • 5 Exhibits only common cause variability Not over-react to minor changes that are part of routine variation

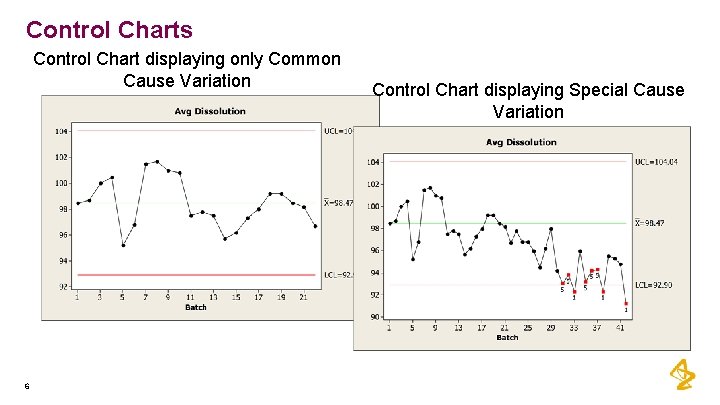
Control Charts Control Chart displaying only Common Cause Variation 6 Control Chart displaying Special Cause Variation

CPV Implementation • Put control of monitoring process in the hands of process owners, not statisticians – this is key! • Make it simple to execute and interpret • Facilitate decision making Requires adjustments to how we implement control charts 7

Control Charts – assumptions • Process stable • Data are normally distributed (for X, X-bar, etc) • Data are identically and independently distributed • Monitored in real time Not often met in pharmaceutical data! 8

Overview • Why use Control Charts? • Type of Data vs Type of Control Chart • Calculating Limits • Responding to Signals - Western Electric Rules • Challenges 9

Data Type vs Control Charts • Data can be continuous or discrete • • • Not all control charts apply to all types of data • 10 Continuous – assay, dissolution, tablet weight Discrete – number of defects, proportion defective But sometimes, we may be able to get away with an “incorrect” chart

Control Charts – Continuous Data • X-Bar and R charts (Normal dist) • Multiple measurements (reported values) per batch • Batch means, range, • Example, tablet weights, dissolution, CU • X and MR chart (Normal dist) • Single measurement (reported value) per batch • Example, water content, p. H, assay 11

Control Charts – Discrete Data • P chart • Proportion of defective units (Binomial dist) • NP chart • Number of defective units (Binomial dist) • C chart • Number of defects (Poisson dist) • U chart • Number of defects per unit (Poisson dist) 12

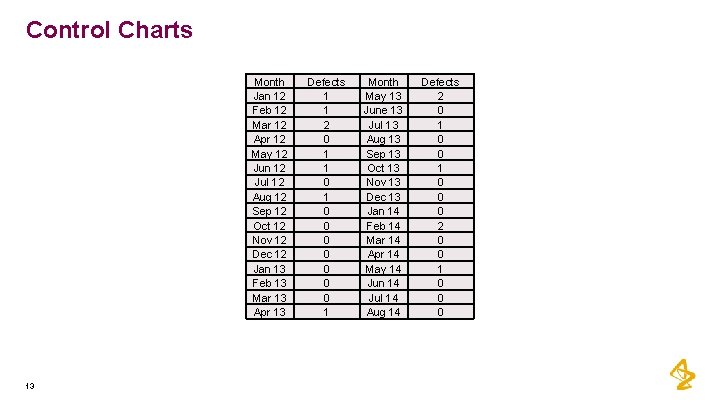
Control Charts Month Jan 12 Feb 12 Mar 12 Apr 12 May 12 Jun 12 Jul 12 Aug 12 Sep 12 Oct 12 Nov 12 Dec 12 Jan 13 Feb 13 Mar 13 Apr 13 13 Defects 1 1 2 0 1 1 0 0 0 0 1 Month May 13 June 13 Jul 13 Aug 13 Sep 13 Oct 13 Nov 13 Dec 13 Jan 14 Feb 14 Mar 14 Apr 14 May 14 Jun 14 Jul 14 Aug 14 Defects 2 0 1 0 0 0 2 0 0 1 0 0 0

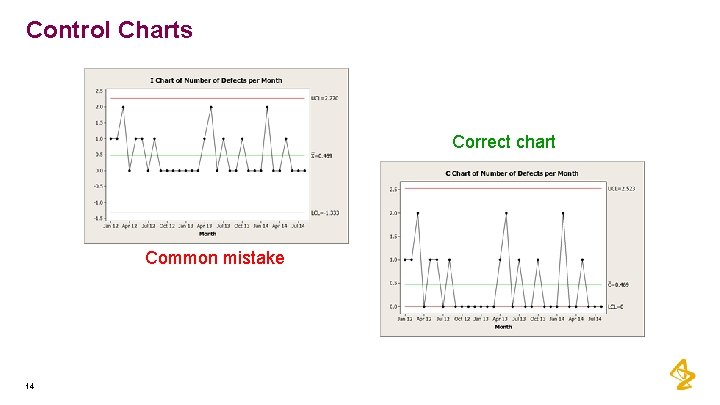
Control Charts Correct chart Common mistake 14

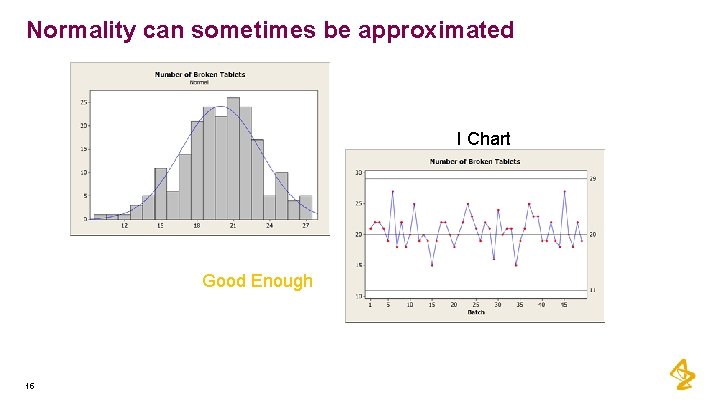
Normality can sometimes be approximated I Chart Good Enough 15

Overview • Why use Control Charts? • Type of Data vs Type of Control Chart • Calculating Limits • Responding to Signals - Western Electric Rules • Challenges 16

Creating Control Charts Calculating Limits • 17 Legacy products • Process history, historical data • Use most recent data – 25 -30 batches • Capture short term and long term variability • Trend data • Is it stable? If not, is there a root cause? • Should any data be excluded? • Some special cause variation is expected • Are there outliers? Should we exclude them?

Creating Control Charts Calculating Limits 18 • Look at histogram • Are data normally distributed? • Are data approximately normally distributed? • Calculate limits based on historical mean and SD

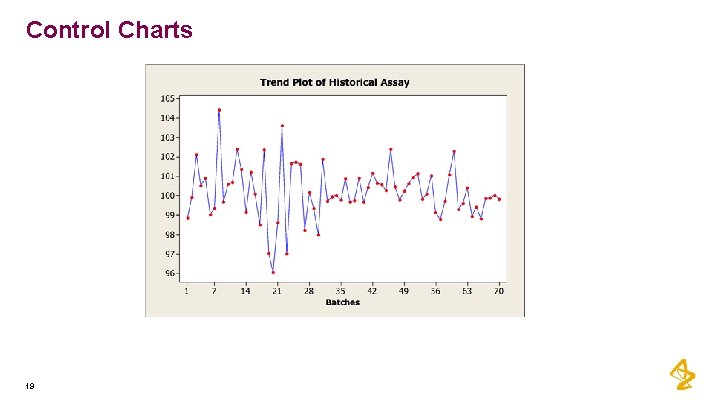
Control Charts 19

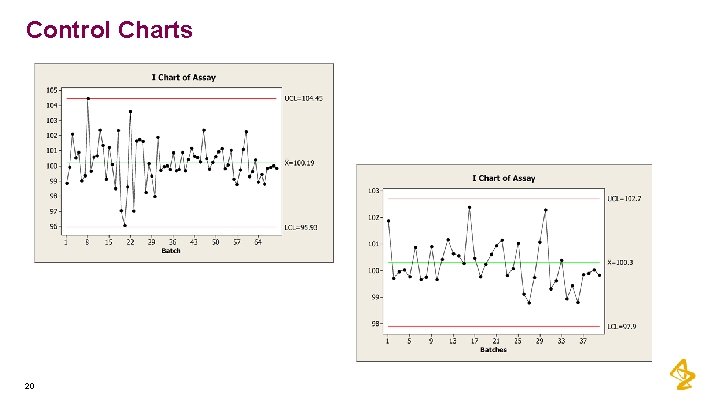
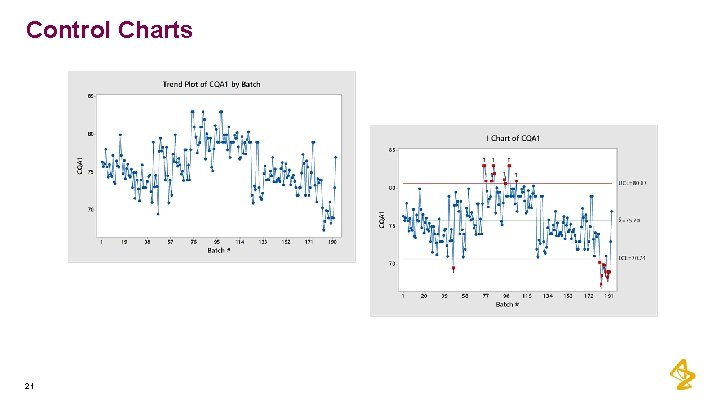
Control Charts 20

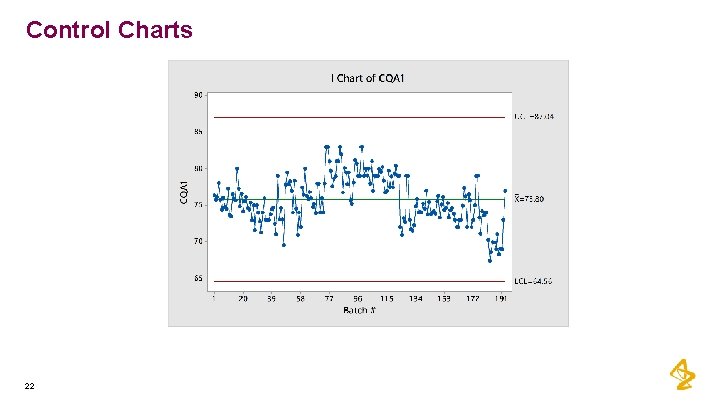
Control Charts 21

Control Charts 22

Control Chart – Assumption of Normality • X-bar, Individual charts – assume normality • Can test for normality but. . . • Test is sensitive to number of samples • Too small => everything will pass normality test • Too large => even known normal data will fail normality if there is an outlier or two • Practical approach – assume normality for tests that are known to be normal, e. g. , assay, CU, tablet weight • Look at histograms to check for extreme/unusual values 23

Control Chart – Assumption of Normality • If data truly non-normal • • • 24 Consider transformation • Interpretation can be difficult Use chart appropriate for underlying distribution, if known Simply trend and track visually • For example, degradant products • Keep specification in mind

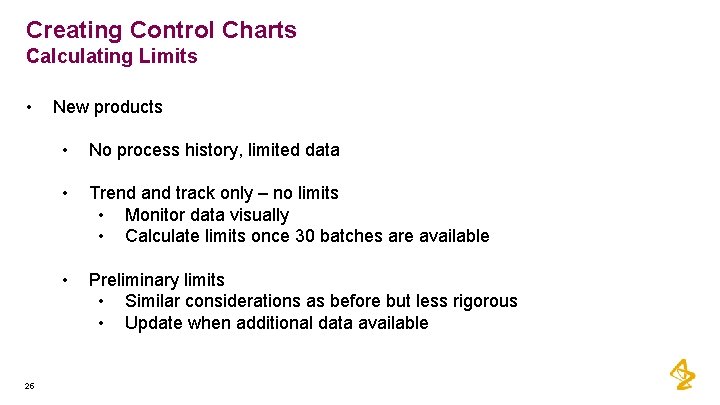
Creating Control Charts Calculating Limits • 25 New products • No process history, limited data • Trend and track only – no limits • Monitor data visually • Calculate limits once 30 batches are available • Preliminary limits • Similar considerations as before but less rigorous • Update when additional data available

Control Charts – Calculating Limits • New products • 26 Common mistake - monitoring current data with limits calculated based on current data!

Overview • Why use Control Charts? • Type of Data vs Type of Control Chart • Calculating Limits • Responding to Signals - Western Electric Rules • Challenges 27

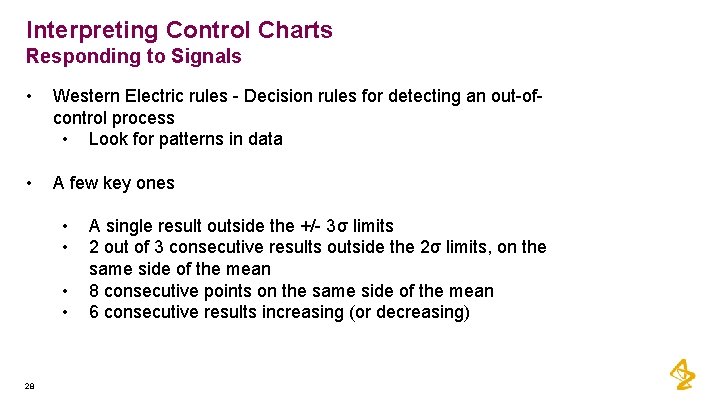
Interpreting Control Charts Responding to Signals • Western Electric rules - Decision rules for detecting an out-ofcontrol process • Look for patterns in data • A few key ones • • 28 A single result outside the +/- 3σ limits 2 out of 3 consecutive results outside the 2σ limits, on the same side of the mean 8 consecutive points on the same side of the mean 6 consecutive results increasing (or decreasing)

• Processes are rarely truly stable • Batch results are rarely independently and identically distributed • Many charts assume underlying normal distribution; data are not always normal 29

• • Batches are often not tested for days after manufacture • Many more batches produced in the interim • Batches tested in order different from manufacturing Signals often observed only during periodic review Limited ability to react in real time! 30

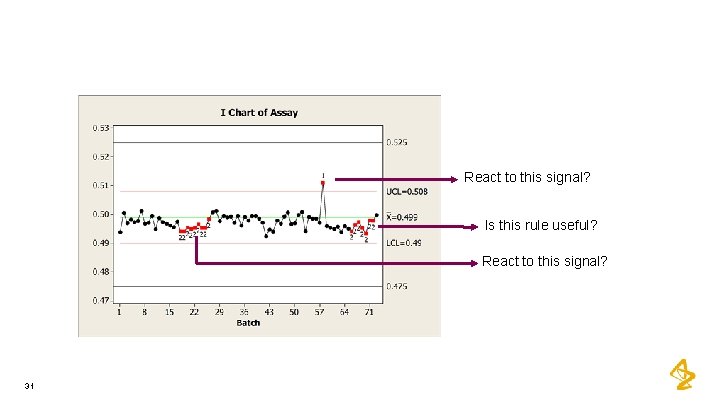
React to this signal? Is this rule useful? React to this signal? 31

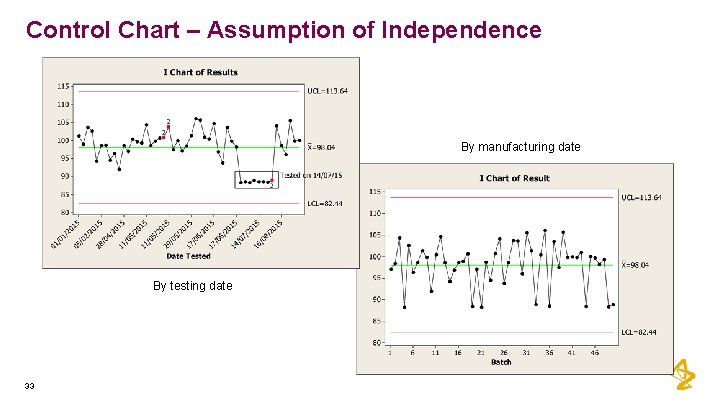
Control Chart – Assumption of Independence • Batches often manufactured in campaigns • Example, based on lots of raw material • Testing often done in groups • Example, multiple batches may be simultaneously tested for assay in the HPLC • May see patterns in data related to above rather than true lackof control 32

Control Chart – Assumption of Independence By manufacturing date By testing date 33

Control Chart – Assumption of Normality • X-bar, Individual charts – assume normality • Can test for normality but. . . • Test is sensitive to number of samples • Too small => everything will pass normality test • Too large => even known normal data will fail normality if there is an outlier or two • Practical approach – assume normality for tests that are known to be normal, e. g. , assay, CU, tablet weight • Look at histograms to check for extreme/unusual values 34

Control Charts – Response to Signals • 35 Risk based approach • Signals should trigger a response • But, response should be commensurate with risk – to patient, process

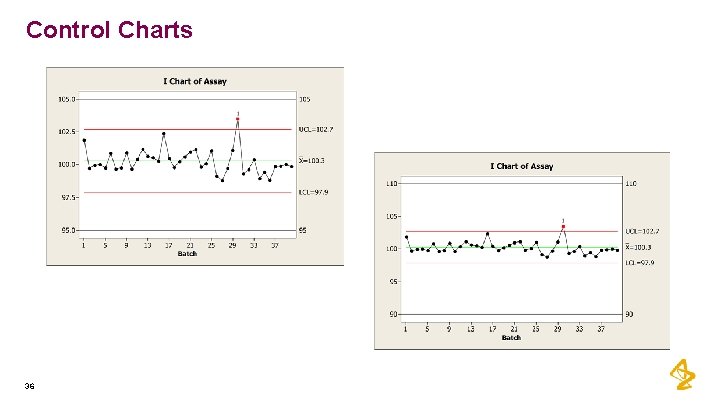
Control Charts 36

Control Charts – Response to Signals • All signals need not be classified as “deviations” • All signals should not lead to full scale investigations • Mindset change for QA organizations 37

Overview • Why use Control Charts? • Type of Data vs Type of Control Chart • Calculating Limits • Responding to Signals - Western Electric Rules • Challenges 38

Challenges • Training provided to staff implementing CPV • Difficult to proceduralize a trained statistician’s thought process and insights • “Number of batches needed” taken too literally • Failure to appreciate limitation of reported (rounded) test results for analysis • Continued coaching / mentoring needed until proficient 39

Summary • Control charts are an important tool for CPV and help us • monitor processes • understand variability • demonstrate that process remains stable and in statistical control • Control chart may need to be looked at differently for pharmaceutical manufacturing • Risk based assessment • Easing of assumptions • Flexibility in implementation 40

Confidentiality Notice This file is private and may contain confidential and proprietary information. If you have received this file in error, please notify us and remove it from your system and note that you must not copy, distribute or take any action in reliance on it. Any unauthorized use or disclosure of the contents of this file is not permitted and may be unlawful. Astra. Zeneca PLC, 1 Francis Crick Avenue, Cambridge Biomedical Campus, Cambridge, CB 2 0 AA, UK, T: +44(0)203 749 5000, www. astrazeneca. com 41