Control by Allostery Enzyme Regulation Glycogen Phosphorylase Control
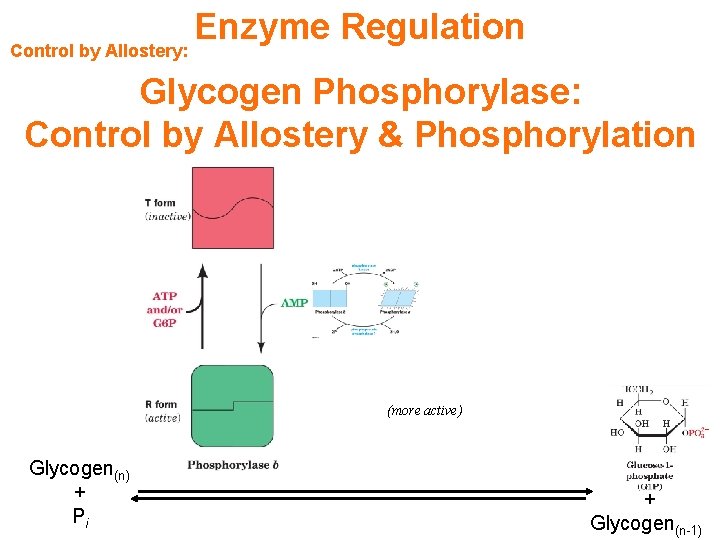
Control by Allostery: Enzyme Regulation Glycogen Phosphorylase: Control by Allostery & Phosphorylation (more active) Glycogen(n) + Pi + Glycogen(n-1)
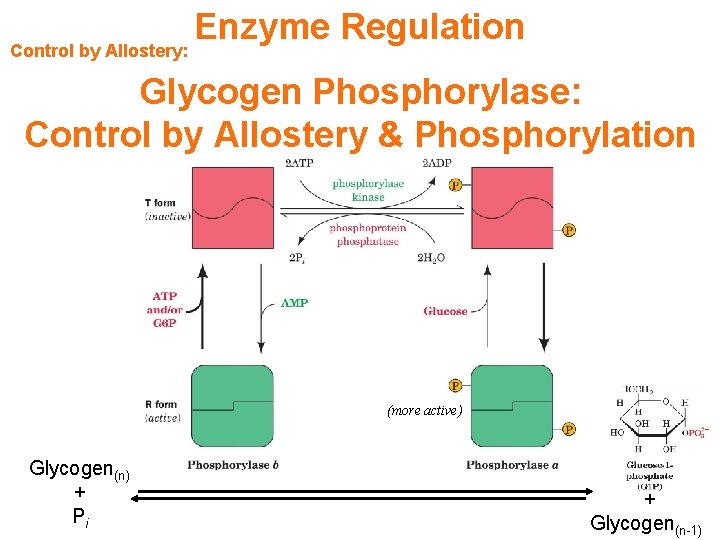
Control by Allostery: Enzyme Regulation Glycogen Phosphorylase: Control by Allostery & Phosphorylation (more active) Glycogen(n) + Pi + Glycogen(n-1)
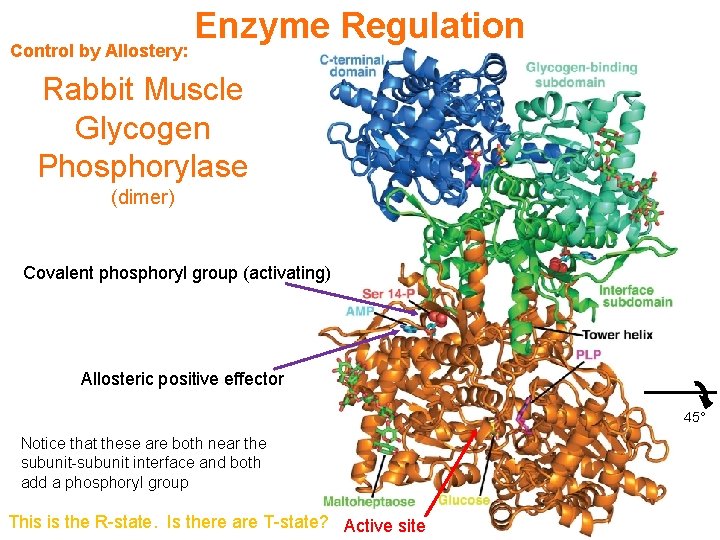
Control by Allostery: Enzyme Regulation Rabbit Muscle Glycogen Phosphorylase (dimer) Covalent phosphoryl group (activating) Allosteric positive effector 45° Notice that these are both near the subunit-subunit interface and both add a phosphoryl group This is the R-state. Is there are T-state? Active site
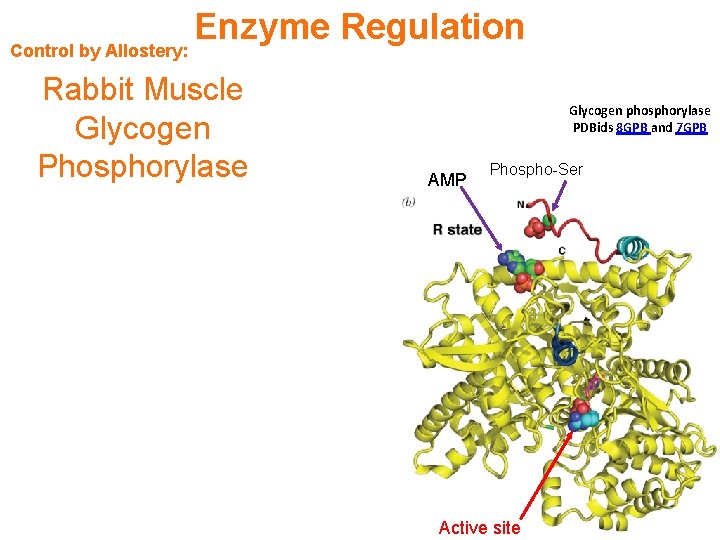
Control by Allostery: Enzyme Regulation Rabbit Muscle Glycogen Phosphorylase Glycogen phosphorylase PDBids 8 GPB and 7 GPB AMP Phospho-Ser Active site
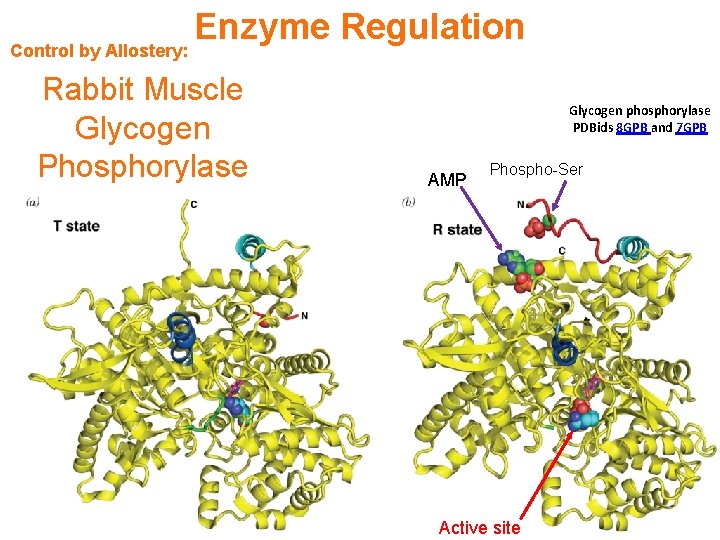
Control by Allostery: Enzyme Regulation Rabbit Muscle Glycogen Phosphorylase Glycogen phosphorylase PDBids 8 GPB and 7 GPB AMP Phospho-Ser Active site
Enzyme Regulation Key Concepts for Control of Enzyme Activity • Allosteric effectors bind to multisubunit enzymes, such as aspartate transcarbamoylase, thereby inducing cooperative conformational changes that alter the enzyme’s catalytic activity. • Phosphorylation and dephosphorylation of an enzyme such as glycogen phosphorylase can control its activity by shifting the equilibrium between more active and less active conformations. You should be able to: • Compare and contrast the actions of an allosteric effector, a competitive enzyme inhibitor, and a noncompetitive inhibitor. • Explain the structural basis for cooperative substrate binding and allosteric control in ATCase. • Why are such allosteric enzymes composed of more than one catalytic subunit? • Describe how phosphorylation and dephosphorylation control the activity of glycogen phosphorylase. • List some advantages of phosphorylation/ dephosphorylation cascade systems over simple allosteric regulation.
- Slides: 6