CONTRACEPTION IN THE FUTURE MODERN CONTRACEPTION AND HOW


































- Slides: 49


CONTRACEPTION IN THE FUTURE MODERN CONTRACEPTION AND HOW TO CHOSE IT

INDIVIDUALISATION IN CONTRACEPTION ?

Endrocrinologist Gregory Pincus (1903 -1967) is best known for developing the oral contraceptive, or birth control pill. THE FIRST OC: In 1960 the compound was approved by the U. S. Food and Drug Administration as the first contraceptive pill. The pill was manufactured by G. D. Searle Company under the name Enovid was sold only by prescription.

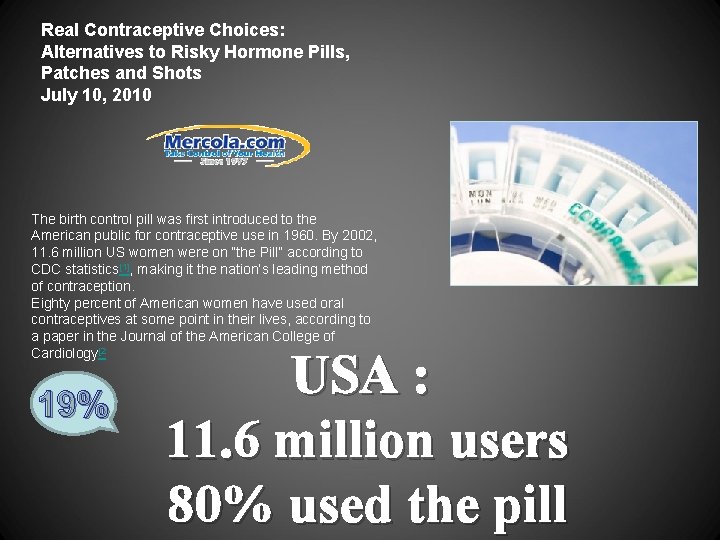
Real Contraceptive Choices: Alternatives to Risky Hormone Pills, Patches and Shots July 10, 2010 The birth control pill was first introduced to the American public for contraceptive use in 1960. By 2002, 11. 6 million US women were on “the Pill” according to CDC statistics[1], making it the nation’s leading method of contraception. Eighty percent of American women have used oral contraceptives at some point in their lives, according to a paper in the Journal of the American College of Cardiology[2 19% USA : 11. 6 million users 80% used the pill

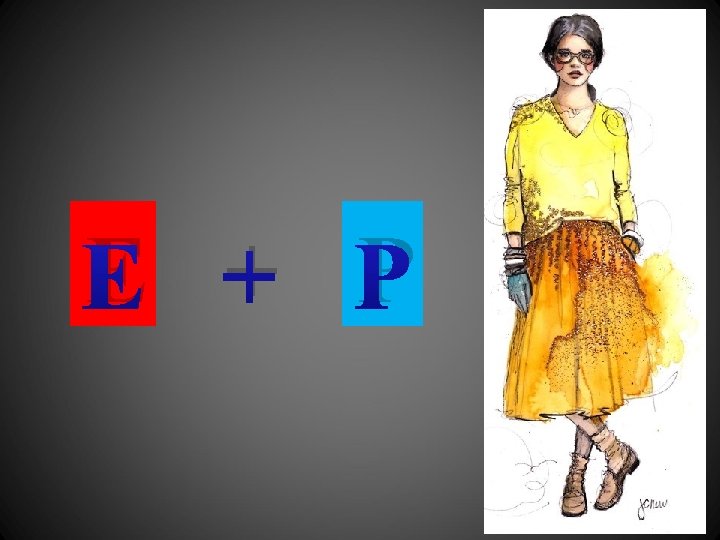
E + P

What side effects could I have whilst taking OC? The vast majority of patients taking OC feel very well but, as with any medicine that has benefits, some minor side effects are occasionally reported. These include: * altered body weight (some gain a few pounds and others lose a little). * nausea and vomiting. * mastalgia (breast tenderness). * headaches (OC should be stopped if they become severe). * altered libido (sex drive - Q 15. 5) with many women noticing an increase and others a reduction. * depression. * reduced or absent menstrual flow. These side effects usually settle within two or three months.

About the pill: In 1960, after more than a decade of research, the United States Food and Drug Administration [USFDA] approved the first oral contraceptive pill, which contained high doses of hormones oestrogen [150 µg] and progestin [9. 85 mg]. These pills satisfied women's need for convenient, safe and reliable contraception. However, some users experienced side effects such as headache, nausea, cramps and weight gain. Though these side effects were temporary, they led to more research and lowering of the hormonal doses in the pills. Today's pills contain around 20 -35 mg of estrogen. This has significantly reduced the side-effects also.

Pill use and smoking: Women who smoke have a higher risk of stroke than non-smokers. Particularly for older women, the combination of oral pills and smoking can increase their risk of stroke and blood clots.

Benefits of the pill Apart from preventing pregnancy, the pills also have other health benefits like: - Regular menstruation - Less severe premenstrual syndrome, dysmenorrhoea - Lowered risk of anaemia [due to lighter menstrual flow] - Protection against some cancers like endometrial and epithelial cancer - Prevention of ectopic pregnancy

• • 1 st pill : 150 mcg EE + 9. 85 P POP – in the 1970 s Gradually diminishing EE dose Switching from EE to natural E (E 2 V and finally 17 -beta-E 2)

The combined oral contraceptive pill has been used by an estimated 300 million women worldwide since it was first developed. In recent years it has been linked with both an increased risk of some forms of cancer and as having a protective effect against some other cancers – leading to considerable confusion for women. Beside that, it has been proven that syntethic E has considerable number of risks as compared to the natural estrogen.

Undesirable side-effects of the classical OCs



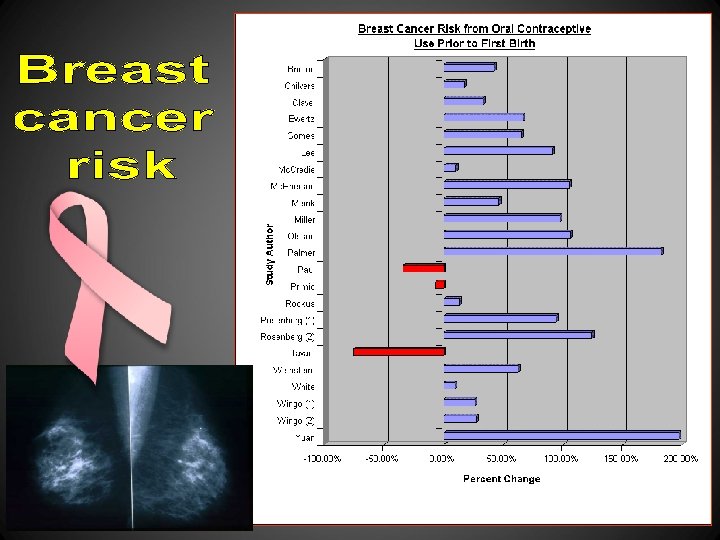
Table 8 A The Four Large Studies That Measured Early OCP Use and the Risk of Breast Cancer to Women Under the Age of 45 Author Year Size of Study Findings Wingo (17) CASH Study 12/80 -82 2089 less than age 45 40% increased risk ages 20 -44 Rosenberg 25* 1977 -1992 1427 less than age 45 88% increased risk** 1983 -1990 50% increased risk: for use within 5 years of 747 less than age 45 menarche (parous women) 5/90 -12/92 1648 less than age 45 White 26 Brinton (19) 42% increased risk (parous women)** * This study did not control for abortion. > ** Calculated from raw data found in their paperÑsee end of chapter for details.

Oral Contraceptives Reduce Long-Term Risk of Ovarian Cancer Since they were first licensed nearly 50 years ago, birth control pills containing estrogen have prevented some 200, 000 cases of ovarian cancer world-wide, estimate the authors of a study published January 26, 2008, in The Lancet. Further, in the absence of having taken oral contraceptives, half of these women would have died of the disease. NCI Cancer Bulletin, vol. 5/no. 3, Feb. 5, 2008 Oral Contraceptives Cut Ovarian Cancer Risk The Pill prevents as many as 30, 000 deaths each year, study says By Steven Reinberg Posted 1/25/08 U. S. National Cancer Institute.

Relation between oral contraceptive hormones and blood clotting Oral contraception with conventional oestrogen/progestogen preparations produces raised levels of clotting factors and increased platelet aggregation. Although these changes do not involve as broad a spectrum of clotting factors as thrombosis and the third trimester of pregnancy, they do not appear a desirable side effect and may be responsible for the increased risk of thrombosis. Oral contraception with progestogen alone does not appear to cause similar clotting and platelet changes. L. Poller , J Clin Pathol Suppl (Assoc Clin Pathol). 1969; 3: 67– 74. Blood Coagulation & Fibrinolysis: July 2002 - Volume 13 - Issue 5 - pp 373 -381 Effect of second- and third-generation oral contraceptives on fibrinolysis in the absence or presence of the factor V Leiden mutation Kemmeren, J. M. ; Algra, A. ; Meijers, J. C. M. ; Bouma, B. N. ; Grobbee, D. E. Third-generation oral contraceptives (OC) have been associated with an increased risk of venous thrombosis compared with second-generation OC. To find an explanation for this increased risk, the effect of a second- and third-generation OC and of the progestagens used in these pills on several fibrinolytic parameters was studied in the absence or presence of the factor V Leiden mutation. In conclusion, the effect of oral contraceptives on fibrinolytic parameters is largely independent of the type of progestagen. The increased fibrinolytic activity during OC use appears to be induced by the estrogen component.

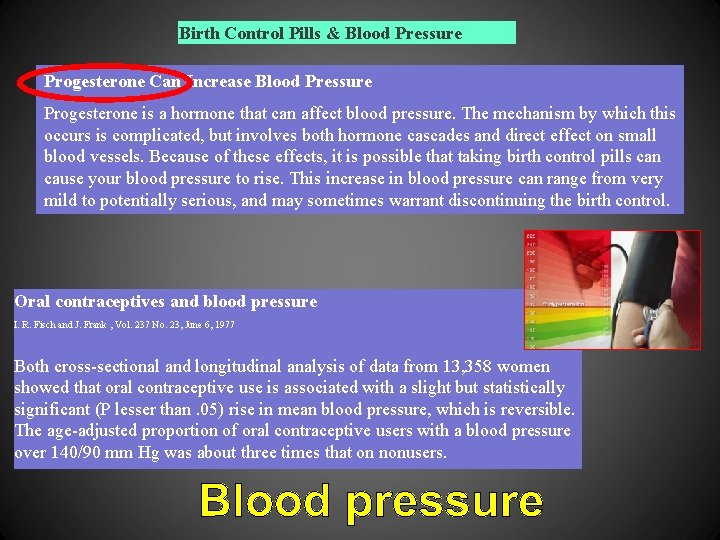
Birth Control Pills & Blood Pressure Progesterone Can Increase Blood Pressure Progesterone is a hormone that can affect blood pressure. The mechanism by which this occurs is complicated, but involves both hormone cascades and direct effect on small blood vessels. Because of these effects, it is possible that taking birth control pills can cause your blood pressure to rise. This increase in blood pressure can range from very mild to potentially serious, and may sometimes warrant discontinuing the birth control. Oral contraceptives and blood pressure I. R. Fisch and J. Frank , Vol. 237 No. 23, June 6, 1977 Both cross-sectional and longitudinal analysis of data from 13, 358 women showed that oral contraceptive use is associated with a slight but statistically significant (P lesser than. 05) rise in mean blood pressure, which is reversible. The age-adjusted proportion of oral contraceptive users with a blood pressure over 140/90 mm Hg was about three times that on nonusers.

Sex hormones and hypertension Raghvendra K. Dubeya, b, *, Suzanne Oparile, Bruno Imthurnb and Edwin K. Jacksona, c, d , Cardiovascular Research 2002 53(3): 688 -708 Estradiol levels increase 50– 180 -fold during pregnancy, and these increases are associated with substantial reductions in blood pressure If endogenous estradiol does lower blood pressure, administration of estrogenic preparations to women might also be expected to reduce blood pressure. However, data on the effects of estrogenic preparations on blood pressure are inconsistent, and include reports of blood pressure lowering, blood pressure elevating, and blood pressure neutral effects. Administration of estradiol (oral or transdermal patches) to normotensive postmenopausal women either reduced or had no effect on blood pressure Contraceptive estrogenic preparations tend to increase blood pressure; conjugated equine estrogens appear to have little effect on blood pressure, and estradiol tends to lower blood pressure Kletzky O. A. , Marrs R. P. , Howard W. F. , Mc. Cormock W. , Mishell D. R. Jr. Prolactin synthesis and release during pregnancy and puerperium. Am J Obstet Gynecol. (1980) 136: 545– 550. Elias A. N. , Meshkinpour H. , Valenta L. J. Attenuation of hypertension by conjugated estrogens. Nephron (1992) 30: 89– 92. WHO Task Force on Oral Contraceptives. The WHO Multicentre Trial of the Vasopressor Effects of Combined Oral Contraceptives. I. Comparisons with IUD. Contraception 1998; 40: 129– 145.

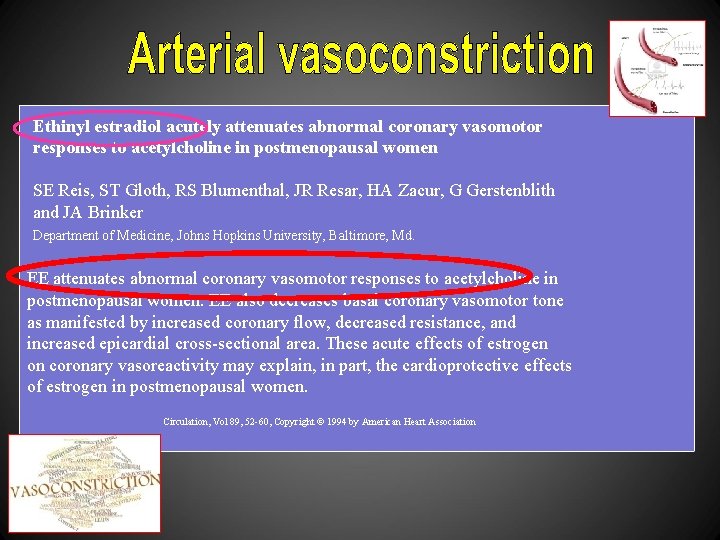
Ethinyl estradiol acutely attenuates abnormal coronary vasomotor responses to acetylcholine in postmenopausal women SE Reis, ST Gloth, RS Blumenthal, JR Resar, HA Zacur, G Gerstenblith and JA Brinker Department of Medicine, Johns Hopkins University, Baltimore, Md. EE attenuates abnormal coronary vasomotor responses to acetylcholine in postmenopausal women. EE also decreases basal coronary vasomotor tone as manifested by increased coronary flow, decreased resistance, and increased epicardial cross-sectional area. These acute effects of estrogen on coronary vasoreactivity may explain, in part, the cardioprotective effects of estrogen in postmenopausal women. Circulation, Vol 89, 52 -60, Copyright © 1994 by American Heart Association

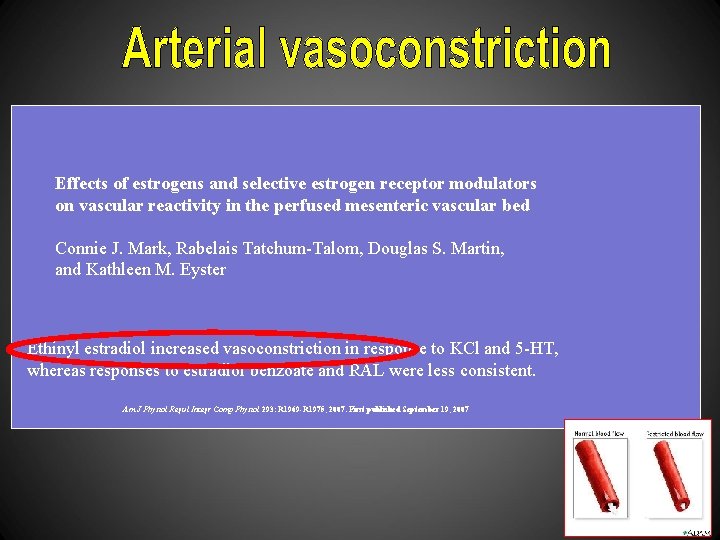
Effects of estrogens and selective estrogen receptor modulators on vascular reactivity in the perfused mesenteric vascular bed Connie J. Mark, Rabelais Tatchum-Talom, Douglas S. Martin, and Kathleen M. Eyster Ethinyl estradiol increased vasoconstriction in response to KCl and 5 -HT, whereas responses to estradiol benzoate and RAL were less consistent. Am J Physiol Regul Integr Comp Physiol 293: R 1969 -R 1975, 2007. First published September 19, 2007

The American Journal of Medicine Volume 49, Issue 5 , Pages 630 -648, November 1970 M. D. Herman Adlercreutz, M. D. Raimo Tenhunen This article deals with some aspects of the interaction between natural and synthetic female sex hormones and the liver. Because of the close structural relationship between some progestogens and anabolic steroids the effect of the latter compounds on the liver is also reviewed. The following effects of anabolic steroids, progesterone, synthetic progestogens, estrogens, compounds with estrogenic activity and oral contraceptives on liver are discussed in greater detail: Effect on morphology including light and electron microscopic changes; effect on bile secretion, with special emphasis on cholestatic effects; effect on biochemistry of the liver cell with special regard to enzyme induction, the drug-metabolizing enzyme system of the liver cell, porphyrin metabolism and changes in mitochondrial enzymes. Jaundice and porphyria due to steroids are also discussed, and the close connection between recurrent (intrahepatic cholestatic) jaundice of pregnancy and jaundice due to oral contraceptives is emphasized. The metabolism of female sex hormones in liver disease is briefly reviewed. In the general discussion the main topics are: Effect of oral contraceptives on the liver, the mechanism of the cholestatic effect of steroids, possible genetical defects involved and the likely connection between the metabolism of hormonal steroids and the formation of gallstones in women.

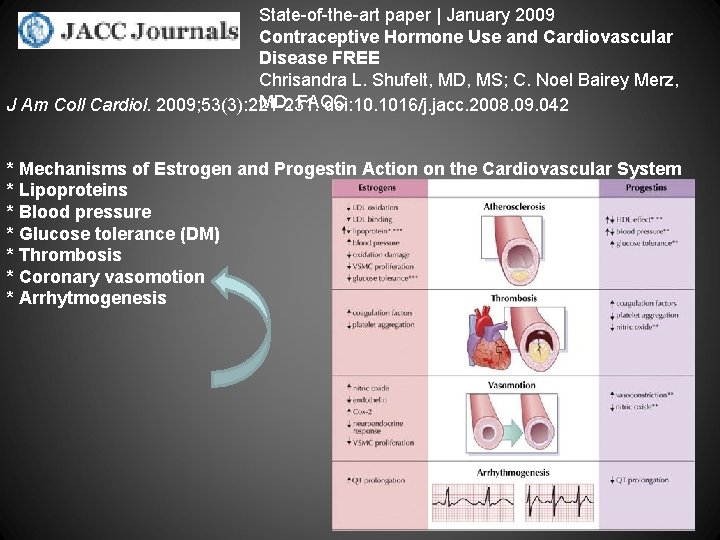
State-of-the-art paper | January 2009 Contraceptive Hormone Use and Cardiovascular Disease FREE Chrisandra L. Shufelt, MD, MS; C. Noel Bairey Merz, MD, FACC doi: 10. 1016/j. jacc. 2008. 09. 042 J Am Coll Cardiol. 2009; 53(3): 221 -231. In the U. S. , hormone therapy delivered as oral contraceptives (OCs) is one of the most commonly prescribed birth control methods, used by 11. 6 million or 19% of women (1). Since their introduction in the 1960 s, OCs have been used by approximately 80% of U. S. women at some point in their life to block ovulation, implantation, and, therefore, pregnancy (2). The simplicity of the available regimens, low frequency of side effects, and relative safety compared with pregnancy (3) has resulted in widespread use. Observational studies demonstrate that young women have a relatively lower age-adjusted risk of cardiovascular disease compared with men. Cardiovascular risk rises after menopause (4), suggesting that endogenous reproductive hormones may play a protective role. We and others have further demonstrated that disruption of ovulatory cycling, indicated by estrogen deficiency and hypothalamic dysfunction (5), or irregular menstrual cycling (6 -7) in pre-menopausal women is associated with an increased risk of coronary atherosclerosis and adverse cardiovascular events, respectively. The concept that pre-menopausal contraceptive hormone use may be protective for atherosclerosis is appealing. Conversely, recently published data on mortality from cardiovascular disease has shown that since the year 2000, mortality rates have increased in women between the ages of 35 and 44 years compared with decreases in all other age groups (4). Increased rates of obesity and smoking and declines in physical activity are prevalent in this group of midlife women (8). Also coincident in this age group was an increased OC use during the same decades, from 4% to 17% (1 -2). In part because OCs are effective and safe for contraception, and because premenopausal women are at relatively lower cardiovascular risk than the general public, there has been relatively little specific study devoted to evaluating links between contraceptive hormone use and cardiovascular disease.

State-of-the-art paper | January 2009 Contraceptive Hormone Use and Cardiovascular Disease FREE Chrisandra L. Shufelt, MD, MS; C. Noel Bairey Merz, MD, FACC J Am Coll Cardiol. 2009; 53(3): 221 -231. doi: 10. 1016/j. jacc. 2008. 09. 042 * Mechanisms of Estrogen and Progestin Action on the Cardiovascular System * Lipoproteins * Blood pressure * Glucose tolerance (DM) * Thrombosis * Coronary vasomotion * Arrhytmogenesis

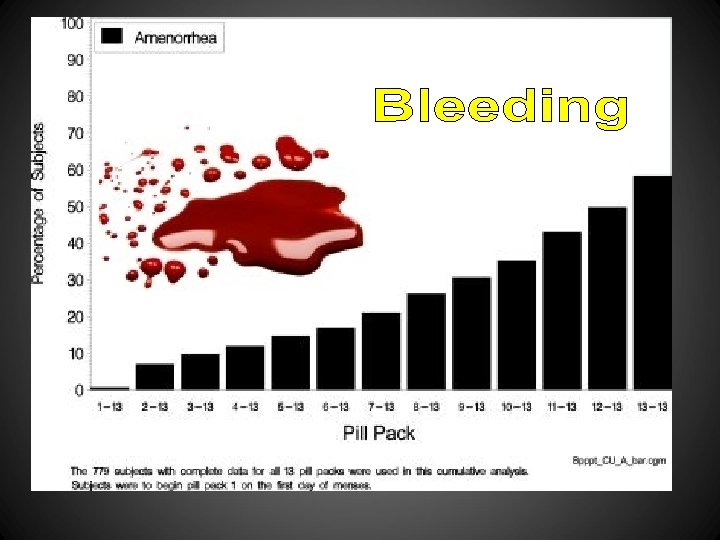
1: Acta Obstet Gynecol Scand. 1981; 60(2): 203 -6. Effectivity and acceptability of oral contraceptives containing natural and artificial estrogens in combination with a gestagen. A controlled double-blind investigation. Serup J, Bostofte E, Larsen S, Westergaard J. With the purpose of investigating effectivity and acceptability of an oral contraceptive containing micronized natural estrogens (estradiol-17 beta 4 mg + estriol 2 mg/norethisterone acetate 3 mg per tablet) versus a contraceptive containing artificial estrogen (ethinyl estradiol 50 micrograms/ norethisterone acetate 3 mg per tablet), a controlled double-blind investigation was performed in 111 young women. The investigation was designed for 12 cycles. The tablets were given consecutively for 3 weeks with 1 week's interruption. In the natural estrogen group 57 women completed 504 cycles, in the artificial estrogen group 54 women completed 510 cycles (Table 1). No pregnancies occurred. There were highly significantly more terminations due to bleeding irregularities (p less than 0. 001), and highly significantly more spotting (p less than 0. 001), breakthrough (p less than 0. 001) and amenorrhea (p less than 0. 001) episodes in the natural estrogen group (Tables II and III). Bleeding irregularities on natural estrogens did not subside during the trial (Table IV). There were a few more psychiatric and CNS symptoms on natural estrogen (p less than 0. 05), but other side effects did not differ between the two preparations (Table V). Blood pressure and weight did not vary significantly. Despite documented metabolic advantages, the natural estrogen tablet investigated was not found to be clinically acceptable for general usage because of the high incidence of bleeding irregularities. It is conceivable that a change of the estrogen/gestagen ratio, using a variable 3 -week schedule, would reduce the number of bleeding irregularities. PIP: With the purpose of investigating effectivity and acceptability of an oral contraceptive containing micronized natural estrogens (estradiol-17 beta 4 mg + estriol 2 mg/norethisterone acetate 3 mg/tablet) versus a contraceptive containing artificial estrogen (ethinyl estradiol 50 mcg/norethisterone acetate 3 mg/tablet), a controlled double-blind investigation was performed in 111 young women. The investigation was designed for 12 cycles. The tablets were given consecutively for 3 weeks with 1 week's interruption. In the natural estrogen group, 57 women completed 504 cycles; in the artificial estrogen group 54 women completed 510 cycles. No pregnancies occurred. There were highly significantly more terminations due to bleeding irregularities (P 0. 001), and highly significantly more spotting (p 0. 001), breakthrough (p 0. 001) and amenorrhea (p 0. 001) episodes in the natural estrogen group. Bleeding irregularities on natural estrogens did not subside during the trial. There were a few more psychiatric and central nervous system symptoms on natural estrogen (p 0. 05), but other side effects did not differ between the 2 preparations. Blood pressure and weight did not vary significantly. Despite documented metabolic advantages, the natural estrogen tablet investigated was not found to be clinically acceptable for general usage because of the high incidence of bleeding irregularities. It is conceivable that a change of the estrogen/gestagen ratio, using a variable 3 -week schedule, would reduce the number of bleeding irregularities. PMID: 7018165 [Pub. Med - indexed for MEDLINE]

Vaginal bleeding patterns among women using one natural and eight hormonal methods of contraception E. M. Belsey Contraception Volume 38, Issue 2 , Pages 181 -206, August 1988 Menstrual diary records were obtained from a total of 5257 women using nine different methods of contraception, one natural and eight hormonal. This paper presents a comparative analysis of their vaginal bleeding patterns. The analytic procedures follow the recommendations of a recent WHO workshop on bleeding pattern analysis, which involve dividing each subject's diary into successive 90 -day reference periods, calculating ten indices for each period, and classifying women according to whether they have “clinically important” bleeding disturbances. In general, the findings of this analysis confirm those of previous studies. Women using the natural method, who were deliberately selected for the regularity of their menstrual cycles, averaged three bleeding/spotting episodes of length 5 days in each 90 -day period, with very little variability within or between women. Subjects given a combined oral contraceptive had more regular patterns than any other treated group, with short (4 -day) episodes and 23– 24 day bleeding-free intervals. Progestogen-only pill users had more frequent, longer episodes and shorter, less predictable intervals than combined pill users. Contrary to widely-held beliefs, the progestogen-only pills produced fewer spotting days than the combined pills, and almost no spotting episodes at all. Nearly half of vaginal ring users experienced some menstrual disturbance in each period; their most common problems were irregular, infrequent or prolonged bleeding. Women using the long-acting injectable, depot medroxyprogesterone acetate, had totally unpredictable patterns, with infrequent but prolonged bleeding/spotting episodes. The incidence of amenorrhea rose from just under 10% in their first injection interval to over 40% in their fourth. The methods of analysis recommended by WHO in 1985 still require substantial refinement. Nevertheless, they are more sensitive than those used previously for WHO trials and produce an easily understood, clinically meaningful characterization of bleeding patterns.

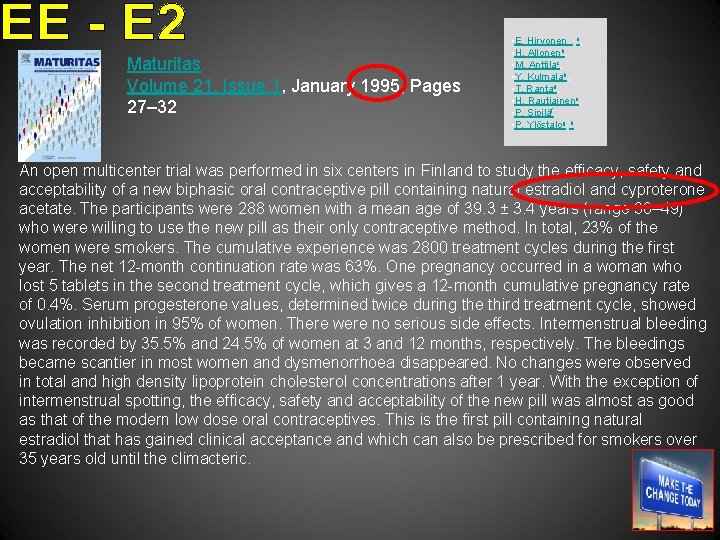
Maturitas Volume 21, Issue 1, January 1995, Pages 27– 32 • E. Hirvonen , a, • H. Allonenb, • M. Anttilac, • Y. Kulmalab, • T. Rantad, • H. Rautiainene, • P. Sipiläf, • P. Ylöstalog, h An open multicenter trial was performed in six centers in Finland to study the efficacy, safety and acceptability of a new biphasic oral contraceptive pill containing natural estradiol and cyproterone acetate. The participants were 288 women with a mean age of 39. 3 ± 3. 4 years (range 30– 49) who were willing to use the new pill as their only contraceptive method. In total, 23% of the women were smokers. The cumulative experience was 2800 treatment cycles during the first year. The net 12 -month continuation rate was 63%. One pregnancy occurred in a woman who lost 5 tablets in the second treatment cycle, which gives a 12 -month cumulative pregnancy rate of 0. 4%. Serum progesterone values, determined twice during the third treatment cycle, showed ovulation inhibition in 95% of women. There were no serious side effects. Intermenstrual bleeding was recorded by 35. 5% and 24. 5% of women at 3 and 12 months, respectively. The bleedings became scantier in most women and dysmenorrhoea disappeared. No changes were observed in total and high density lipoprotein cholesterol concentrations after 1 year. With the exception of intermenstrual spotting, the efficacy, safety and acceptability of the new pill was almost as good as that of the modern low dose oral contraceptives. This is the first pill containing natural estradiol that has gained clinical acceptance and which can also be prescribed for smokers over 35 years old until the climacteric.

Experimental and Toxicologic Pathology Volume 50, Issues 4– 6, September 1998, Pages 458– 464 Approaches to the replacement of ethinylestradiol by natural 17β-estradiol in combined oral contraceptives Dedicated to Prof. Dr. Wolfgang Klinger on occasion of his 65 th birthday on July 3, 1998. • H. Hoffmann 1, , • C. Moore 1, • H. Zimmermann 1, • W. Elger 2, • S. Schwarz 1, • T. Gräser 1, • M. Oettel 1 The strong hepatic estrogenic actions of ethinylestradiol (EE) are very likely to be the cause of the cardiovascular morbidity related to the use of combined oral contraceptives (COCs). This survey presents results of EE replacement in. COCs with natural 17β-estradiol (E 2) in the following stages: reduction of EE to daily doses of 0. 01 mg and concomitant replacement with E 2 (as valerate, EV), complete replacement of EE with E 2 using a novel multiphasic combination containing EV and the progestin dienogest (DNG), and the use of natural E 2 to develop estrogen sulfamates (J 995) showing sufficient dissociation of uterine from liver estrogenicity. Recent data from preclinical and clinical studies show that these approaches seem to be promising.

06/17/2009 Bioidentical birth control 2009 "Bayer Schering Pharma, the pharmaceutical giant that makes the YAZ contraceptive pill, claims that they have developed the first ‘natural’ birth control pill in the world. They have already launched the pill, Qlaira, in the UK after doing tests with it on 3, 000 women. Instead of using synthetic hormones, like those that are found in other contraceptive pills, Qlaira uses bioidentical oestrogen hormones from natural plants, which is identical to the estrogen in the body. " There's a bit more if you follow the link, including this sequence of sentences: "Although bioidentical hormones are boasted as safer than synthetic hormones, there is some scientific evidence that supports theory. In January, Suzanne Somers appeared on The Oprah Winfrey show, crediting bioidentical hormones with helping her get through menopause. " I thought that was pretty hilarious. Anyway, this will be an interesting development to keep an eye on. I wonder if there is potential for less side effects, since the hormones are identical to those in our own bodies. Read more at Vagina. Pagina: http: //vaginapagina. livejournal. com/16388054. html#ixzz 2 az 7 ju 8 x 3

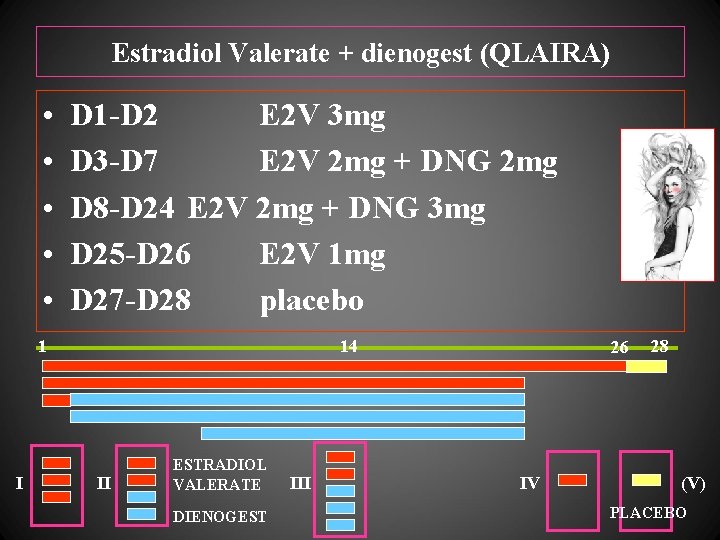
Estradiol Valerate + dienogest (QLAIRA) • • • D 1 -D 2 E 2 V 3 mg D 3 -D 7 E 2 V 2 mg + DNG 2 mg D 8 -D 24 E 2 V 2 mg + DNG 3 mg D 25 -D 26 E 2 V 1 mg D 27 -D 28 placebo 1 I 14 II ESTRADIOL VALERATE DIENOGEST III 26 IV 28 (V) PLACEBO

EV (estradiol valerate) …………………. E 2 (natural estradiol)

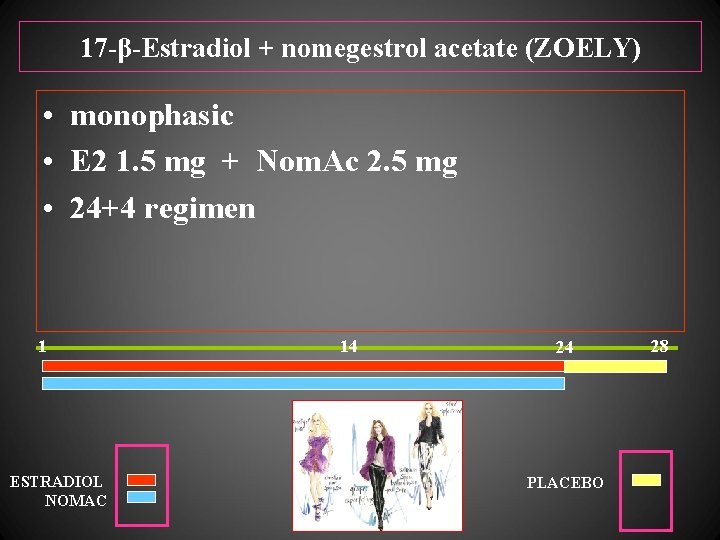
17 -β-Estradiol + nomegestrol acetate (ZOELY) • monophasic • E 2 1. 5 mg + Nom. Ac 2. 5 mg • 24+4 regimen 1 ESTRADIOL NOMAC 14 24 PLACEBO 28

Duration (days) of bleeding in the RVS (Intent-To-Treat Population)

Mean change in SHBG after 3 cycles of treatment with E 2/NOMAC (24 -day regimen)

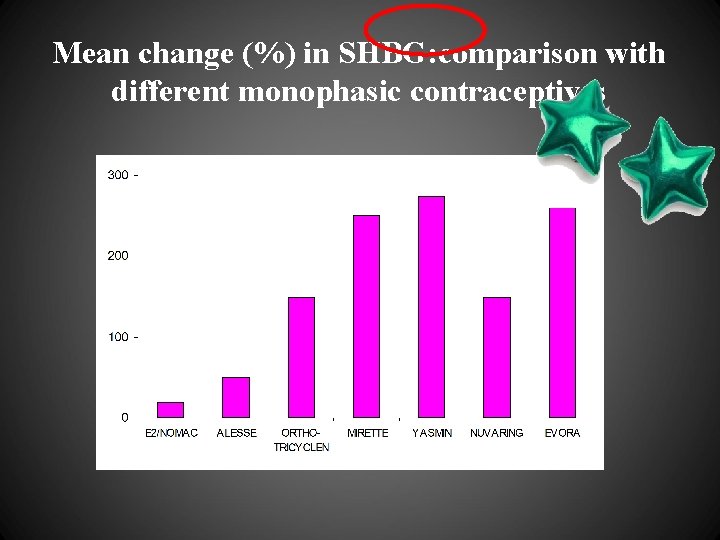
Mean change (%) in SHBG: comparison with different monophasic contraceptives

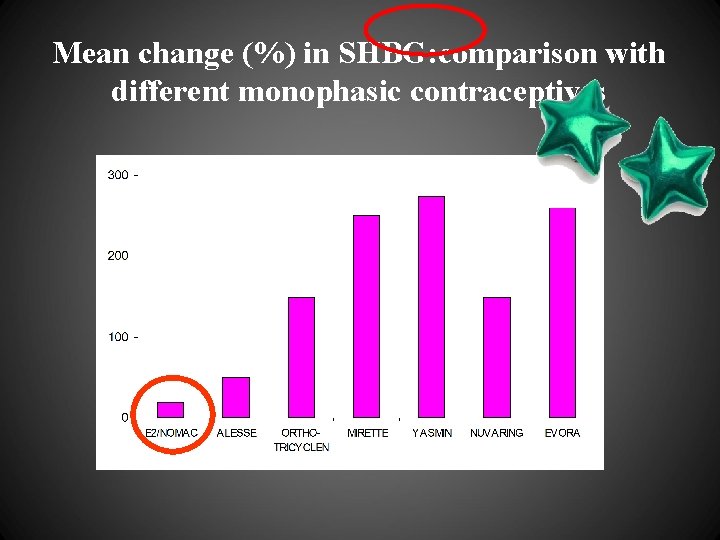
Mean change (%) in SHBG: comparison with different monophasic contraceptives

od Gest O est Dienog ad V LE m Chlor g Deso Nestorone Trimeges NOMAC Drospir NETA ro p y C g no e i D MPA

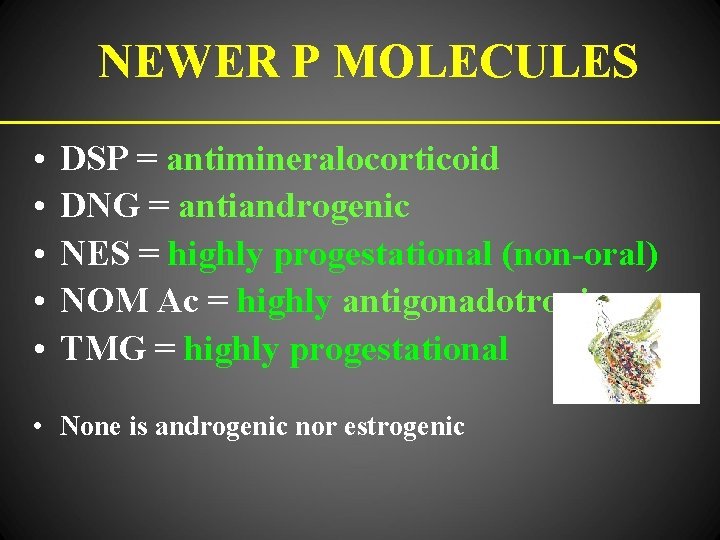
NEWER P MOLECULES • • • DSP = antimineralocorticoid DNG = antiandrogenic NES = highly progestational (non-oral) NOM Ac = highly antigonadotropic TMG = highly progestational • None is androgenic nor estrogenic

GREAT NUMBER OF ADVANTAGES EXIST WHEN IN ORAL CONTRACEPTION EE IS REPLACED WITH NATURAL ESTROGEN! (we have been waiting for it almost 50 years!)

CONTRACEPTION IN THE FUTURE TRANSDERMAL CONTRACEPTION (GEL; E 2 + NESTORONE) MODERN CONTRACEPTION AND HOW TO CHOSE IT

CONTRACEPTION IN THE FUTURE TRANSDERMAL CONTRACEPTION (GEL; E 2 + NESTORONE) E 4 (estetrol) MODERN CONTRACEPTION AND HOW TO CHOSE IT

CONCLUSIONS: CONTRACEPTION WITH NATURAL HORMONES MAY HAVE LESS SIDE EFFECTS

CONCLUSIONS: CONTRACEPTION WITH NATURAL HORMONES MAY HAVE LESS SIDE EFFECTS CONTRACEPTIVE EFFECT DEPENDS MORE ON PROGESTIN (IMPORTANCE OF CHOOSING THE MOST METABOLIC-FRIENDLY ONE!)

CONCLUSIONS: CONTRACEPTION WITH NATURAL HORMONES MAY HAVE LESS SIDE EFFECTS CONTRACEPTIVE EFFECT DEPENDS MORE ON PROGESTIN (IMPORTANCE OF CHOOSING THE MOST METABOLIC-FRIENDLY ONE!) TODAY - E 2 TOMORROW MAYBE E 4

CONCLUSIONS: CONTRACEPTION WITH NATURAL HORMONES MAY HAVE LESS SIDE EFFECTS CONTRACEPTIVE EFFECT DEPENDS MORE ON PROGESTIN (IMPORTANCE OF CHOOSING THE MOST METABOLIC-FRIENDLY ONE!) TODAY - E 2 TOMORROW MAYBE E 4 FUTURE IMPROVEMENTS – TRANSDERMAL ADMINISTRATION (GEL)

Over 50 years of oral contraception … …today, first users are grandmothers!

