Continuous symmetry and chirality Applications across the natural











![The chirality measure of the electron density function of [8]annulene Ch. Dryzun The chirality measure of the electron density function of [8]annulene Ch. Dryzun](https://slidetodoc.com/presentation_image_h/32635e905845f516c8864dc297904207/image-24.jpg)




![“Graying” the Woodward-Hofmann rules: Reactivity-symmetry correlations in Diels-Alder [4+2] reactions Inbal Tuvi-Arad, Chem. Europ. “Graying” the Woodward-Hofmann rules: Reactivity-symmetry correlations in Diels-Alder [4+2] reactions Inbal Tuvi-Arad, Chem. Europ.](https://slidetodoc.com/presentation_image_h/32635e905845f516c8864dc297904207/image-49.jpg)


- Slides: 67

Continuous symmetry and chirality: Applications across the natural sciences David Avnir Institute of Chemistry, The Hebrew University, Jerusalem The Open University, Raanana, 16. 12. 15

1. Motivation







Chirality: Definitions (intuitive) and properties * Chirality: # The property of having for the same object a left-form and a right-form # The property of not having reflection or inversion symmetry * This left and right forms are called enantiomers * The enantiomers are mirror-images of each other * The object and its mirror mage do not coincide


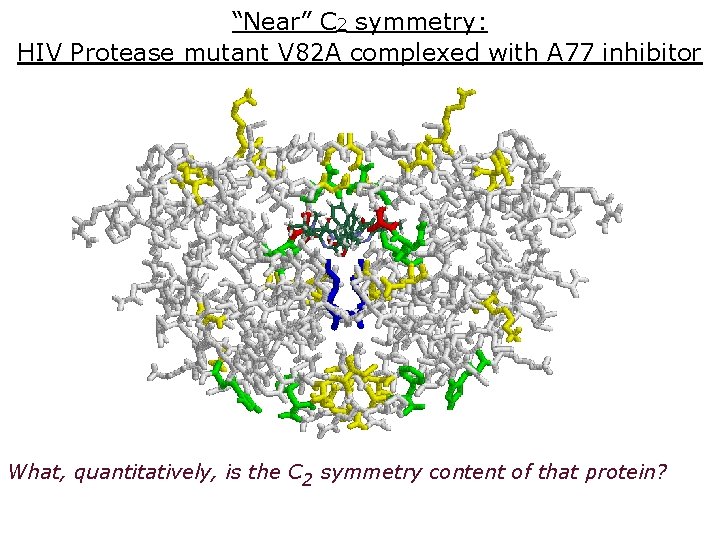
“Near” C 2 symmetry: HIV Protease mutant V 82 A complexed with A 77 inhibitor What, quantitatively, is the C 2 symmetry content of that protein?

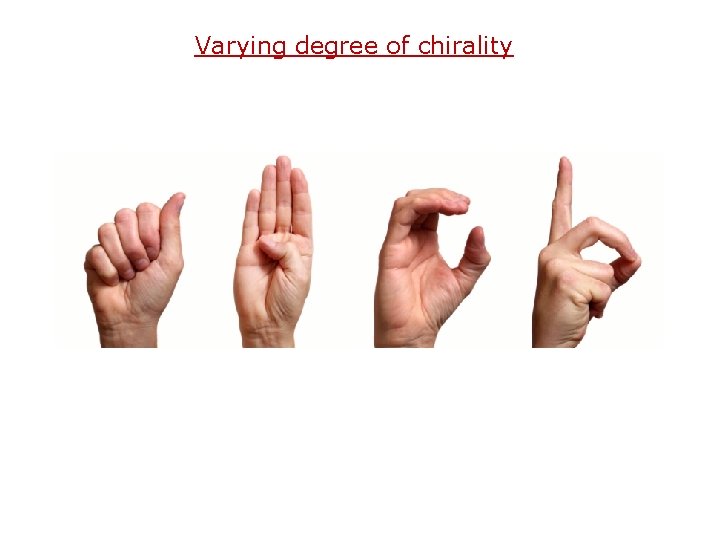
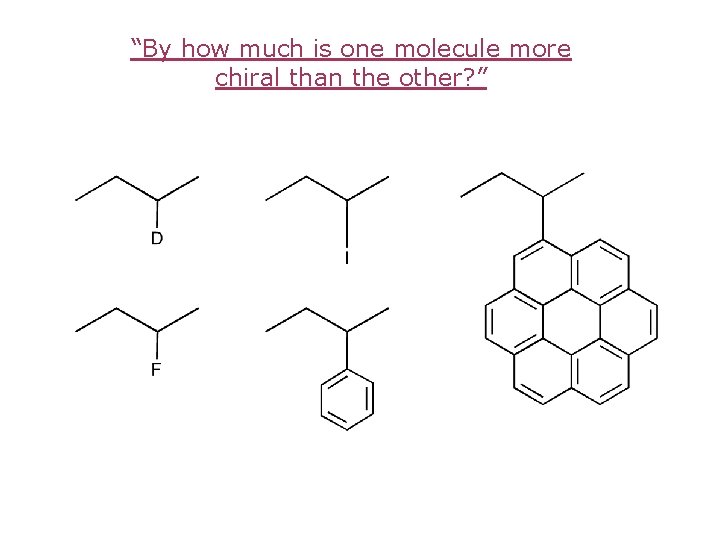
Varying degree of chirality

“By how much is one molecule more chiral than the other? ”

Thanks Hagit Zabrodsky Hel-Or Mark Pinsky Chaim Dryzun Dina Yogev-Einot Inbal Tuvi-Arad Maayan Bonjack Omer Katzenelson Hadassah Elgavi Shahar Keinan Yael Shpigler Sagiv Barhoom Itay Zandbank Barcelona: Santiago Alvarez , Pere Alemany Germany: Robert Berger

2. The continuous symmetry and chirality measures (CSM, CCM)

In designing a measurement of symmetry and chirality, we want it to be able to: # follow gradually changes in deviation from symmetry and chirality in dynamical systems; # be able compare the near symmetry and chirality of different molecules or conformers on the same scale; # translate properly correlations between physical and chemical variables and the “degree of symmetry” or the “degree of chirality”

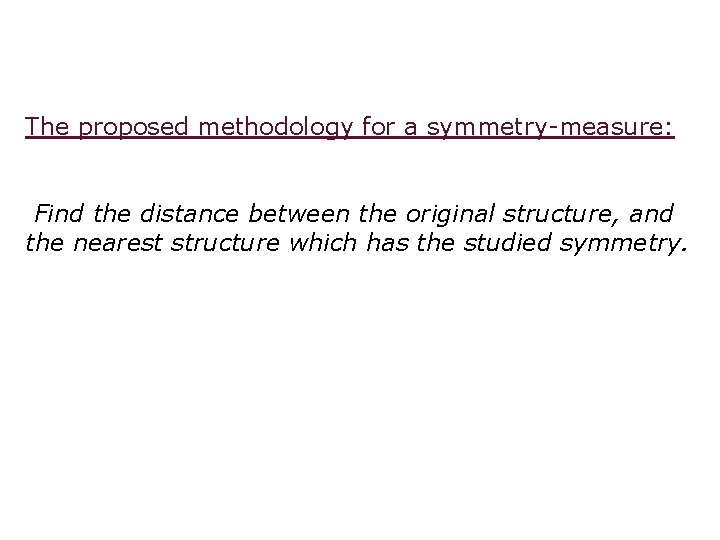
The proposed methodology for a symmetry-measure: Find the distance between the original structure, and the nearest structure which has the studied symmetry.

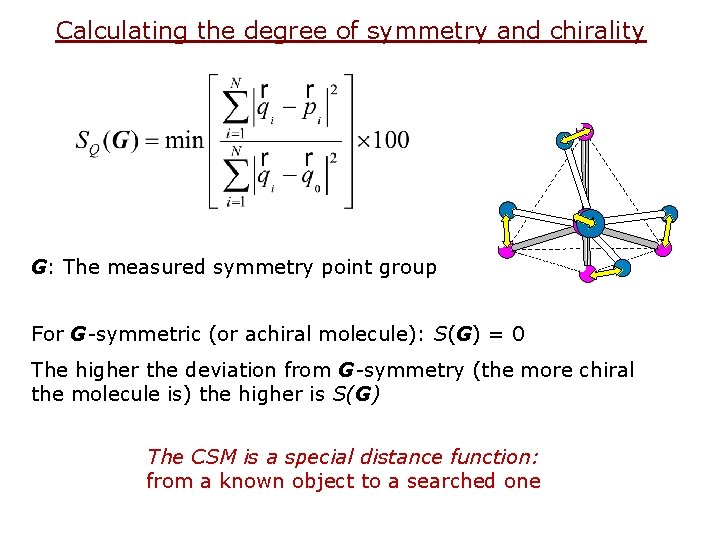
Calculating the degree of symmetry and chirality G: The measured symmetry point group For G-symmetric (or achiral molecule): S(G) = 0 The higher the deviation from G-symmetry (the more chiral the molecule is) the higher is S(G) The CSM is a special distance function: from a known object to a searched one

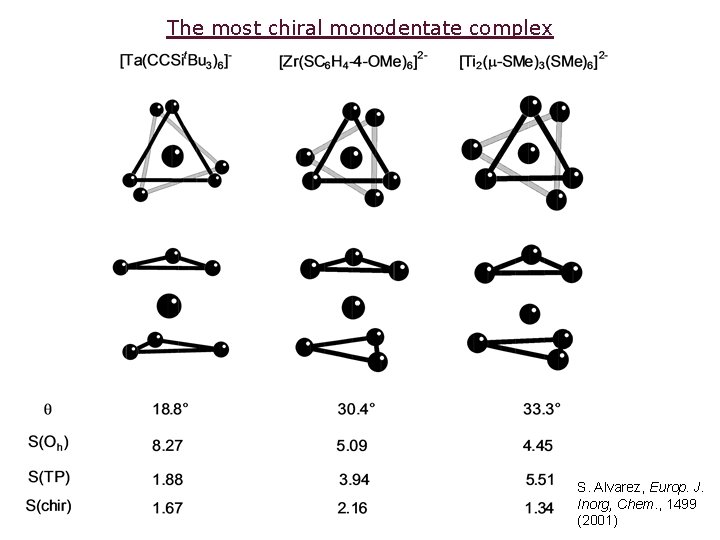
The most chiral monodentate complex S. Alvarez, Europ. J. Inorg, Chem. , 1499 (2001)

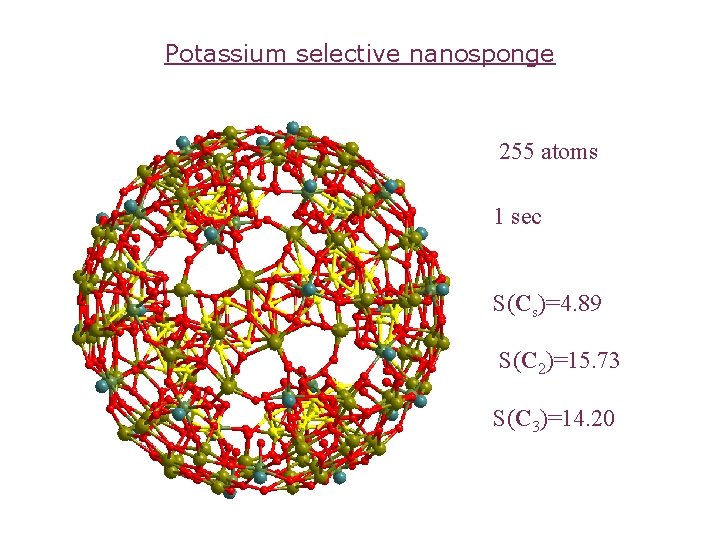
Potassium selective nanosponge 255 atoms 1 sec S(Cs)=4. 89 S(C 2)=15. 73 S(C 3)=14. 20

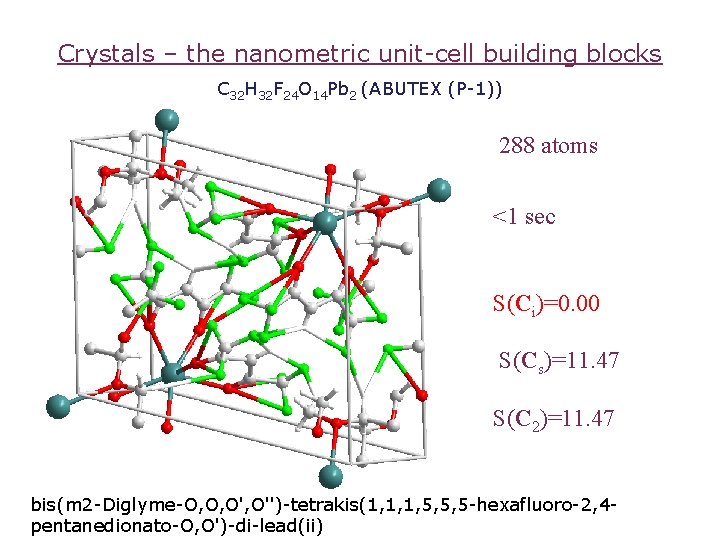
Crystals – the nanometric unit-cell building blocks C 32 H 32 F 24 O 14 Pb 2 (ABUTEX (P-1)) 288 atoms <1 sec S(Ci)=0. 00 S(Cs)=11. 47 S(C 2)=11. 47 bis(m 2 -Diglyme-O, O, O'')-tetrakis(1, 1, 1, 5, 5, 5 -hexafluoro-2, 4 pentanedionato-O, O')-di-lead(ii)

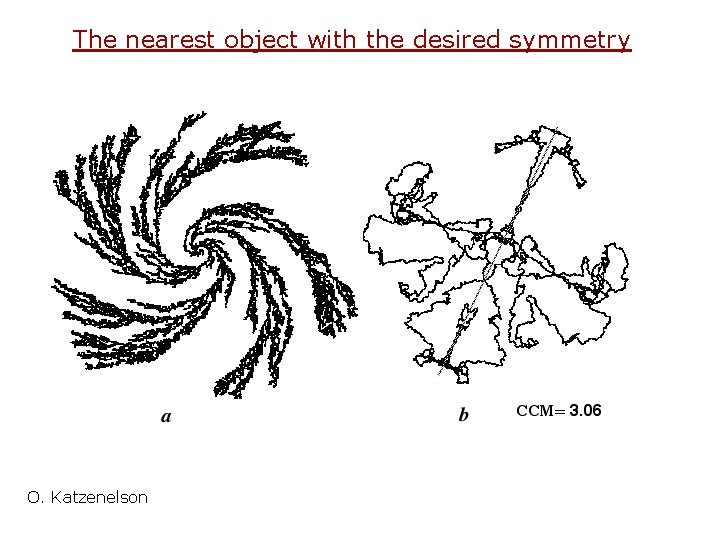
The nearest object with the desired symmetry O. Katzenelson

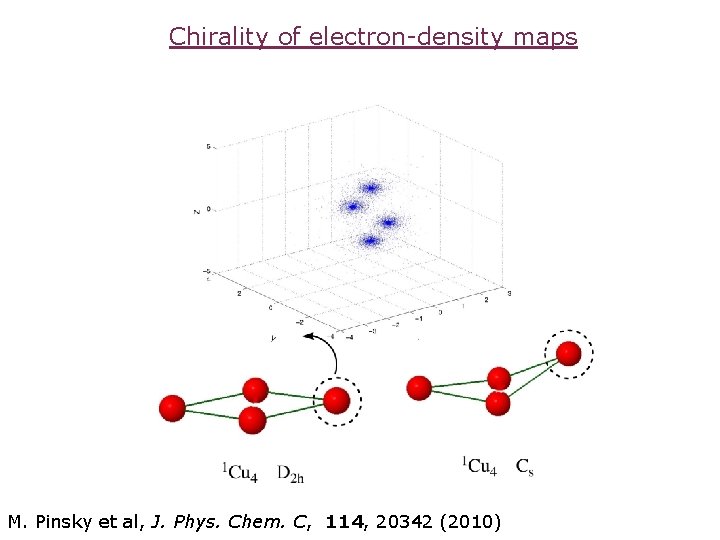
Chirality of electron-density maps M. Pinsky et al, J. Phys. Chem. C, 114, 20342 (2010)
![The chirality measure of the electron density function of 8annulene Ch Dryzun The chirality measure of the electron density function of [8]annulene Ch. Dryzun](https://slidetodoc.com/presentation_image_h/32635e905845f516c8864dc297904207/image-24.jpg)
The chirality measure of the electron density function of [8]annulene Ch. Dryzun

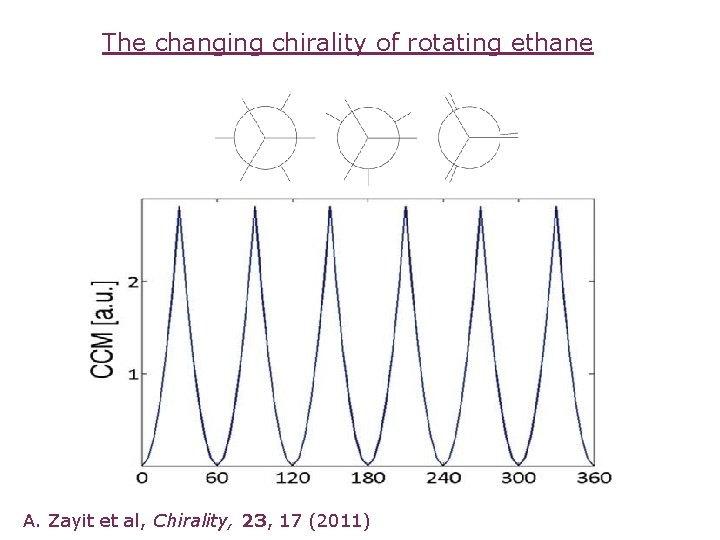
The changing chirality of rotating ethane A. Zayit et al, Chirality, 23, 17 (2011)

3. Applications: Geology Dina Yogev-Einot

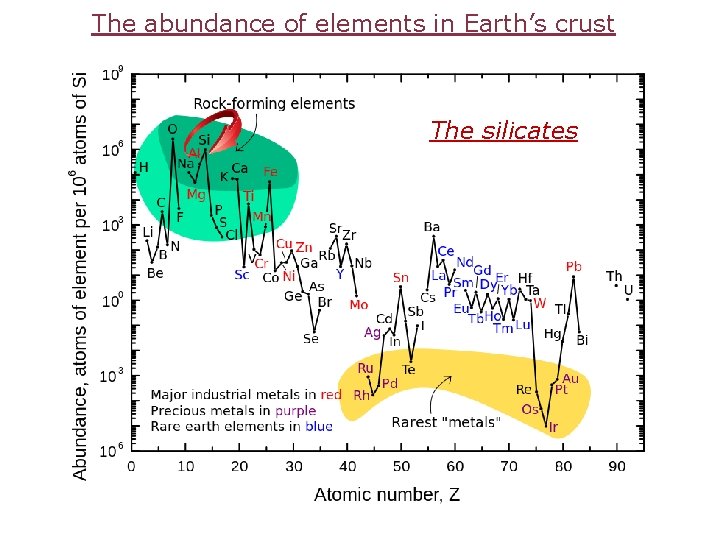
The abundance of elements in Earth’s crust The silicates

The most common mineral in Earth’s crust Quartz = 59. 7 (%weight) Feldspar = 15. 4 Haematite = 2. 6 Mg. O = 4. 4 Quartz is chiral (https: //answers. yahoo. com/question/index? qid=20121205130239 AAPOhoq )

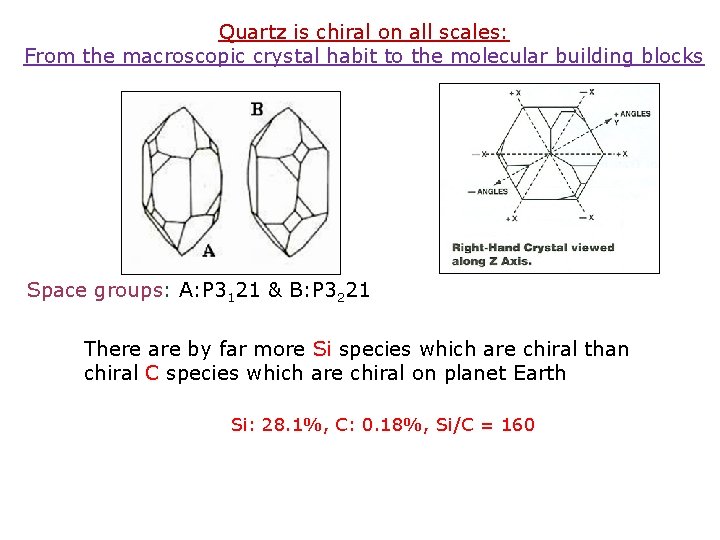
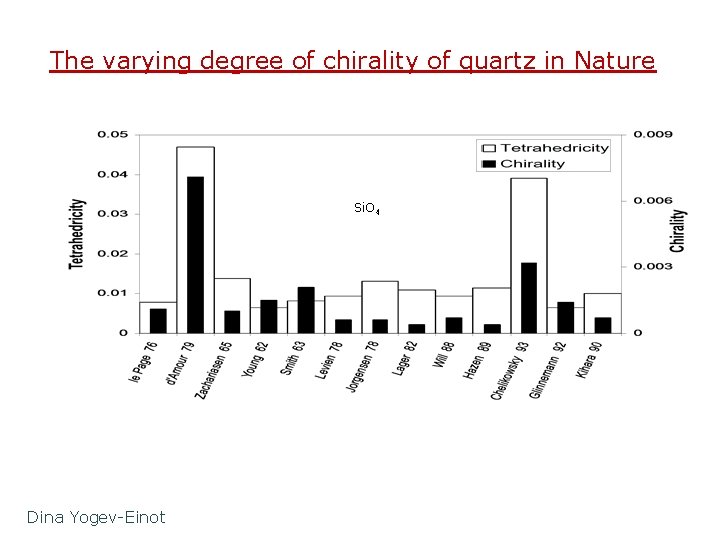
Quartz is chiral on all scales: From the macroscopic crystal habit to the molecular building blocks Space groups: A: P 3121 & B: P 3221 There are by far more Si species which are chiral than chiral C species which are chiral on planet Earth Si: 28. 1%, C: 0. 18%, Si/C = 160

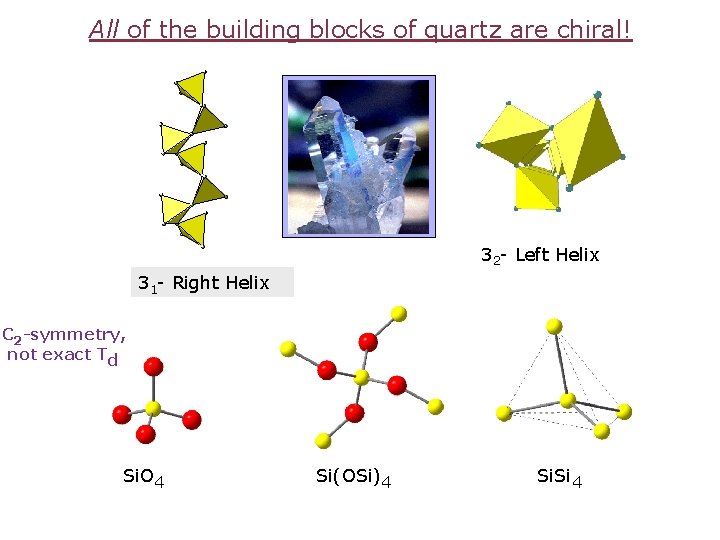
All of the building blocks of quartz are chiral! 32 - Left Helix 31 - Right Helix C 2 -symmetry, not exact Td Si. O 4 Si(OSi)4 Si. Si 4

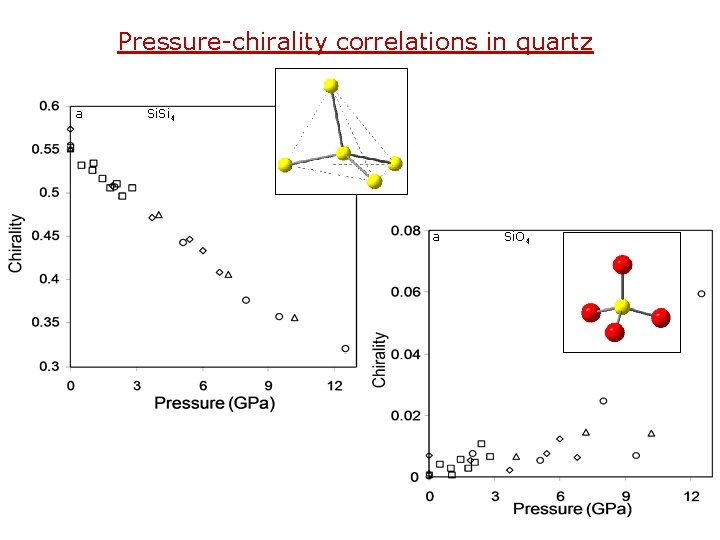
Pressure-chirality correlations in quartz a Si. Si 4 a Si. O 4

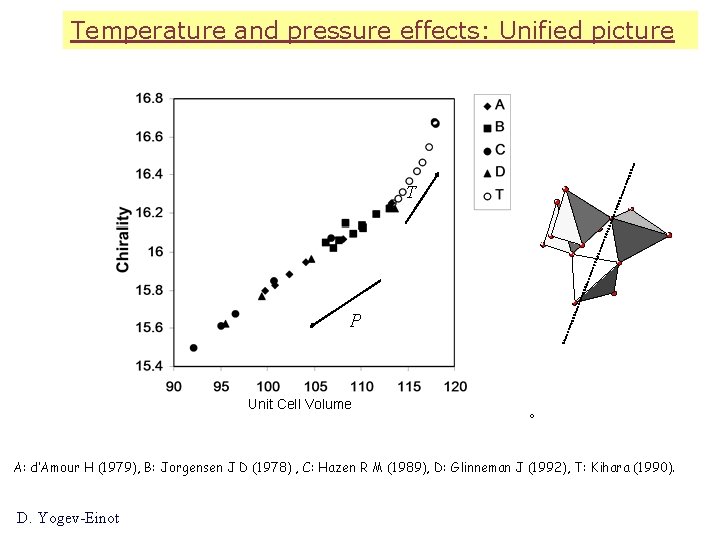
Temperature and pressure effects: Unified picture T P Unit Cell Volume A: d’Amour H (1979), B: Jorgensen J D (1978) , C: Hazen R M (1989), D: Glinneman J (1992), T: Kihara (1990). D. Yogev-Einot

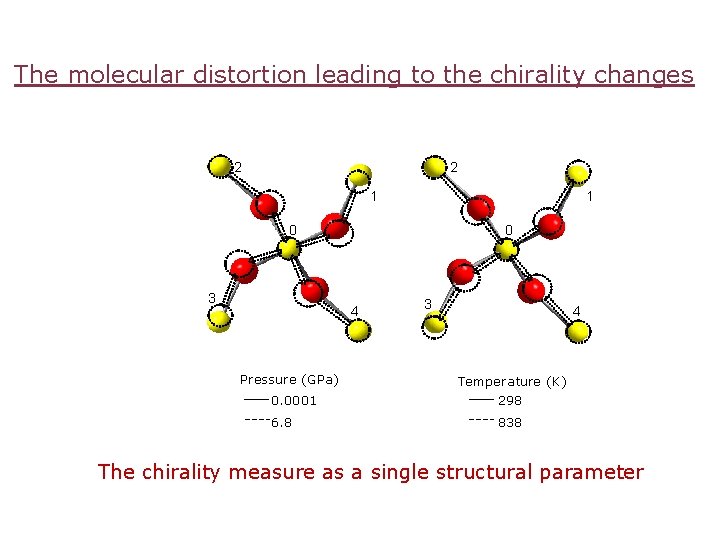
The molecular distortion leading to the chirality changes 2 2 1 1 0 3 0 4 Pressure (GPa) 0. 0001 6. 8 3 4 Temperature (K) 298 838 The chirality measure as a single structural parameter

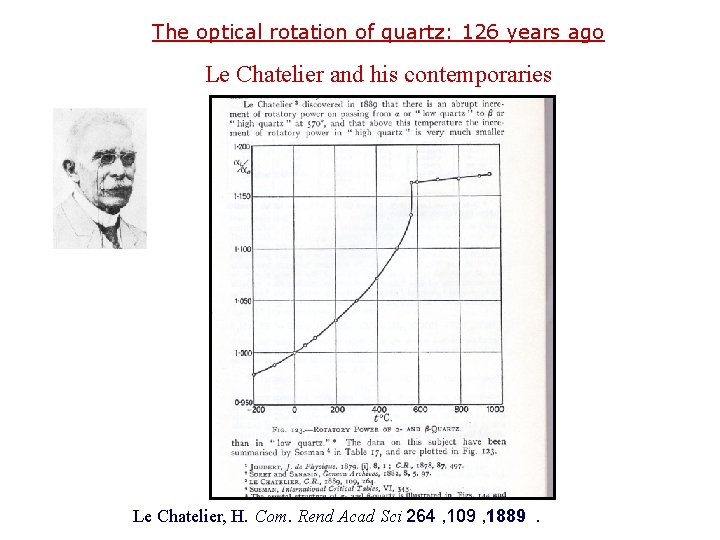
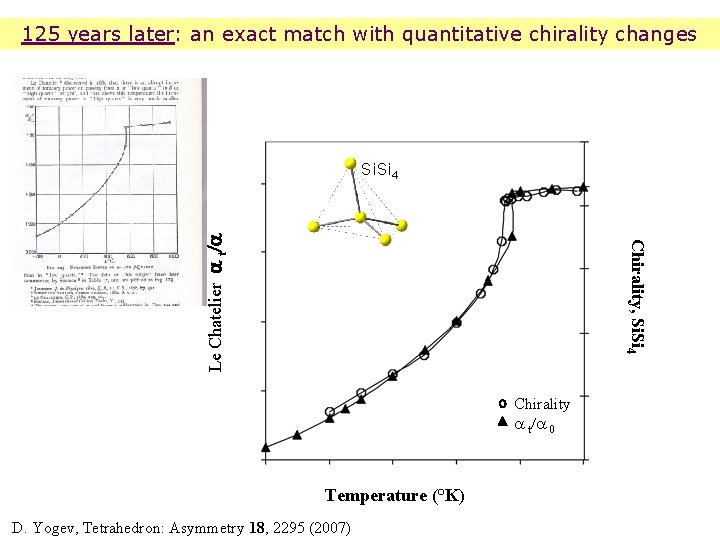
The optical rotation of quartz: 126 years ago Le Chatelier and his contemporaries Le Chatelier, H. Com. Rend Acad Sci 264 , 109 , 1889.

125 years later: an exact match with quantitative chirality changes Chirality, Si. Si 4 Le Chatelier a t/a Si. Si 4 Chirality a t/a 0 Temperature (°K) D. Yogev, Tetrahedron: Asymmetry 18, 2295 (2007)

The varying degree of chirality of quartz in Nature Si. O 4 Dina Yogev-Einot

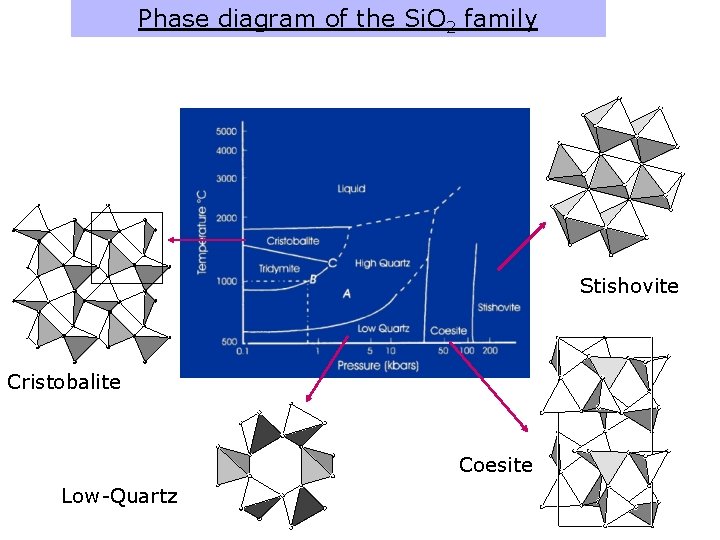
Phase diagram of the Si. O 2 family Stishovite Cristobalite Coesite Low-Quartz

4. Applications: Biology

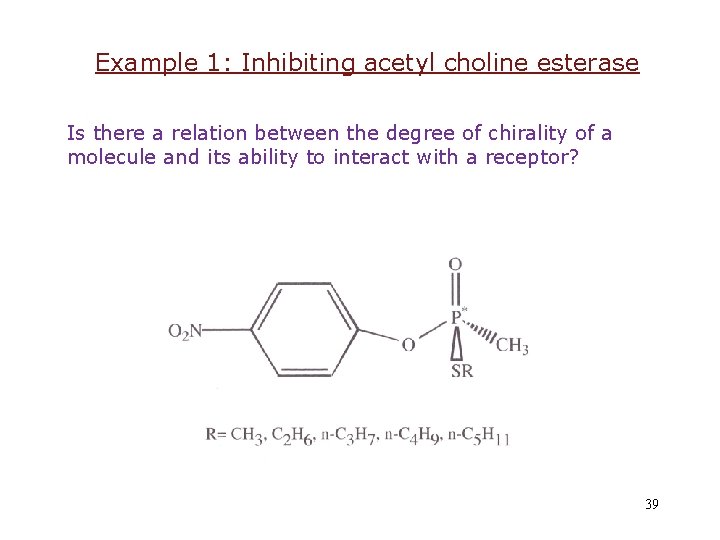
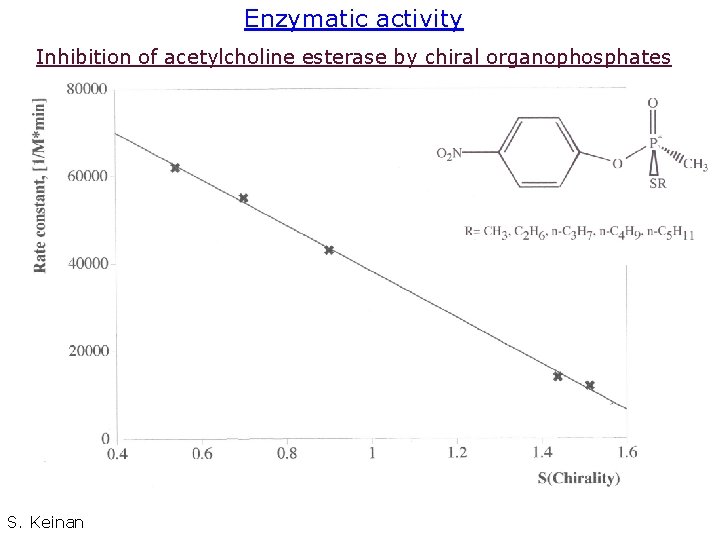
Example 1: Inhibiting acetyl choline esterase Is there a relation between the degree of chirality of a molecule and its ability to interact with a receptor? 39

Enzymatic activity Inhibition of acetylcholine esterase by chiral organophosphates S. Keinan

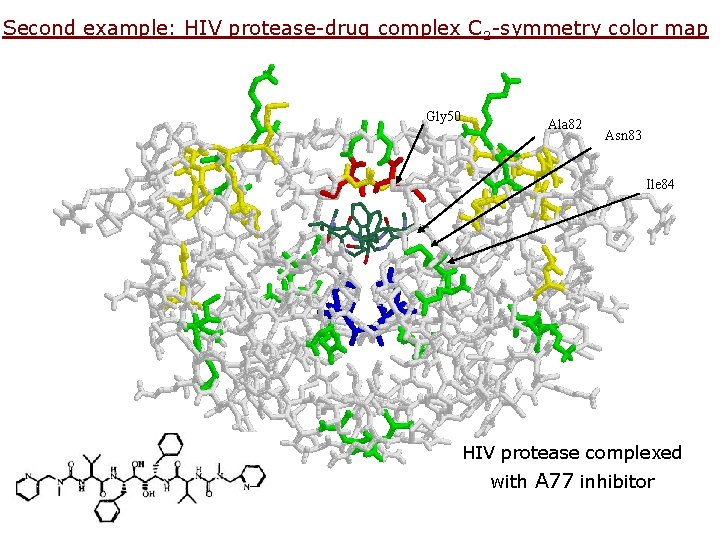
Second example: HIV protease-drug complex C 2 -symmetry color map Gly 50 Ala 82 Asn 83 Ile 84 HIV protease complexed with A 77 inhibitor

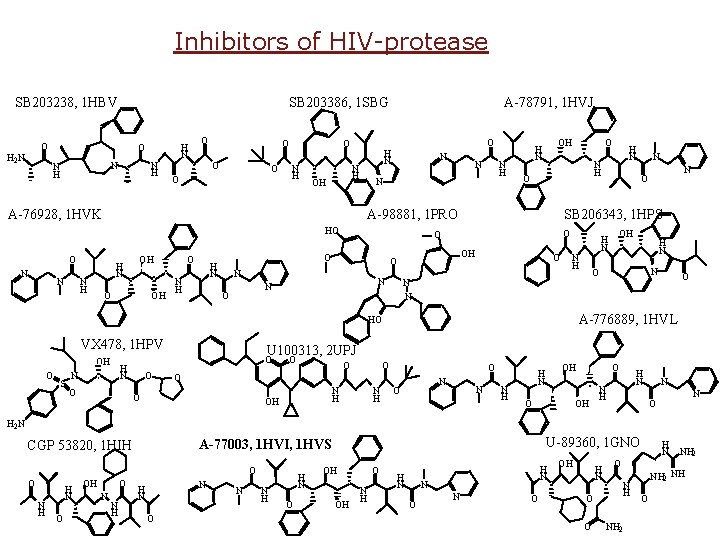
Inhibitors of HIV-protease SB 203238, 1 HBV SB 203386, 1 SBG O H 2 N O N H N N H H N O O O N H A-76928, 1 HVK O H N N H OH A-78791, 1 HVJ N N O N H N N H OH O O N H N N N O O OH O S N O O N OH H N N O A-776889, 1 HVL U 100313, 2 UPJ O H N N H HO VX 478, 1 HPV H N SB 206343, 1 HPS O O H N O N H A-98881, 1 PRO HO N N H N OH H N O O O N H OH N O N N H OH H N O O OH H N N O H 2 N O O N H H N O OH O N N H U-89360, 1 GNO A-77003, 1 HVI, 1 HVS CGP 53820, 1 HIH N O N N H H N O OH OH O N H H N N O OH H N O O H N O N H NH 2 NH O

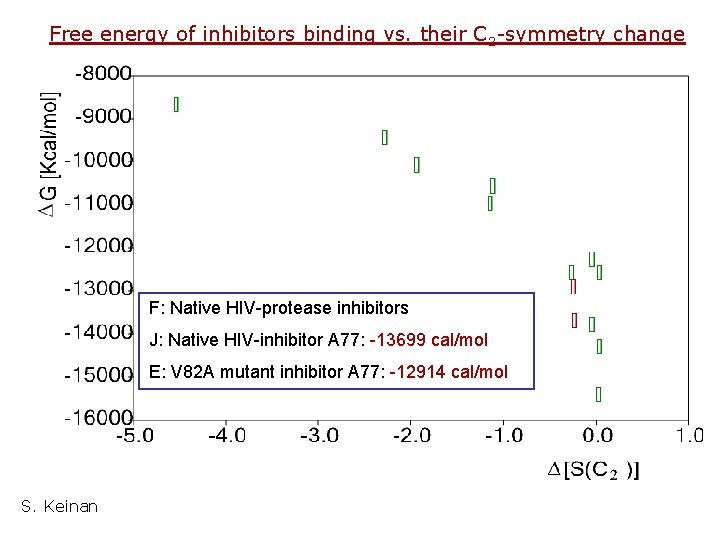
Free energy of inhibitors binding vs. their C 2 -symmetry change F: Native HIV-protease inhibitors J: Native HIV-inhibitor A 77: -13699 cal/mol E: V 82 A mutant inhibitor A 77: -12914 cal/mol S. Keinan

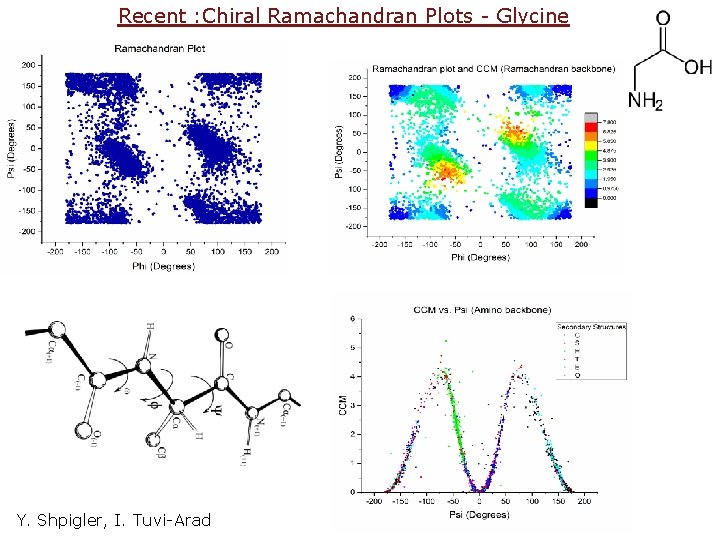
Recent : Chiral Ramachandran Plots - Glycine Y. Shpigler, I. Tuvi-Arad

5. Applications: Chemistry

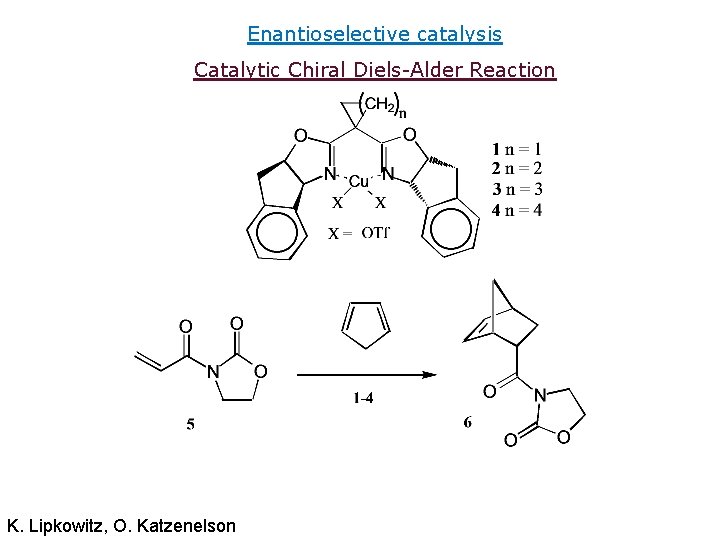
Enantioselective catalysis Catalytic Chiral Diels-Alder Reaction K. Lipkowitz, O. Katzenelson

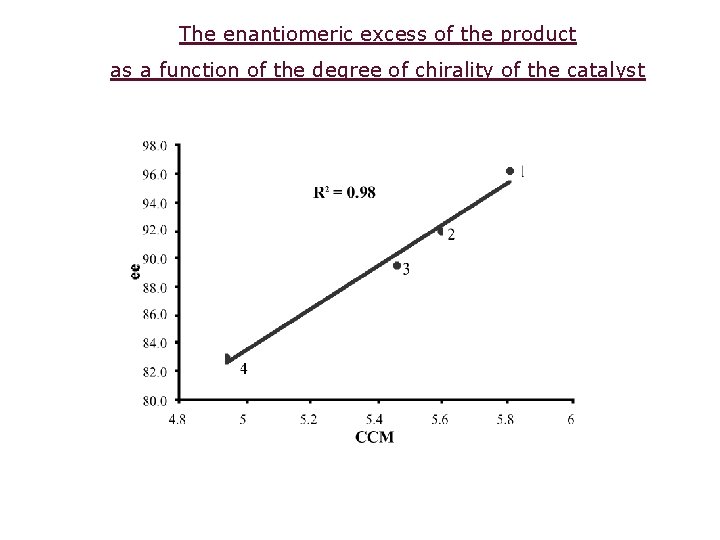
The enantiomeric excess of the product as a function of the degree of chirality of the catalyst

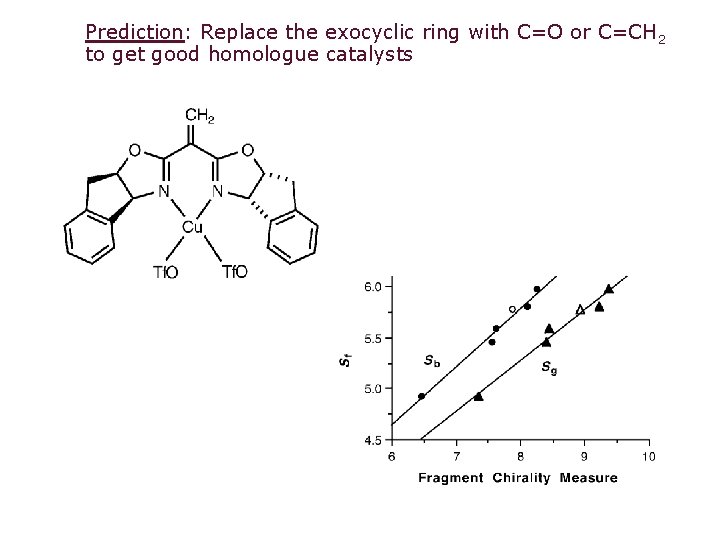
Prediction: Replace the exocyclic ring with C=O or C=CH 2 to get good homologue catalysts
![Graying the WoodwardHofmann rules Reactivitysymmetry correlations in DielsAlder 42 reactions Inbal TuviArad Chem Europ “Graying” the Woodward-Hofmann rules: Reactivity-symmetry correlations in Diels-Alder [4+2] reactions Inbal Tuvi-Arad, Chem. Europ.](https://slidetodoc.com/presentation_image_h/32635e905845f516c8864dc297904207/image-49.jpg)
“Graying” the Woodward-Hofmann rules: Reactivity-symmetry correlations in Diels-Alder [4+2] reactions Inbal Tuvi-Arad, Chem. Europ. J. , 2012, 18, 10014

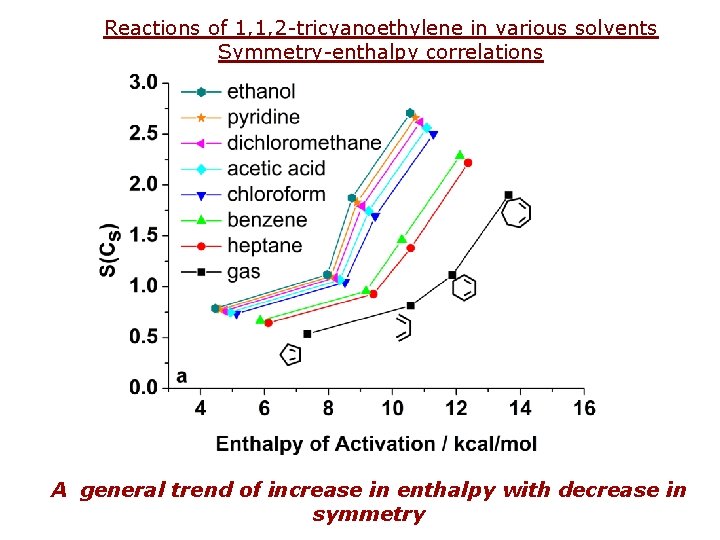
Reactions of 1, 1, 2 -tricyanoethylene in various solvents Symmetry-enthalpy correlations A general trend of increase in enthalpy with decrease in symmetry

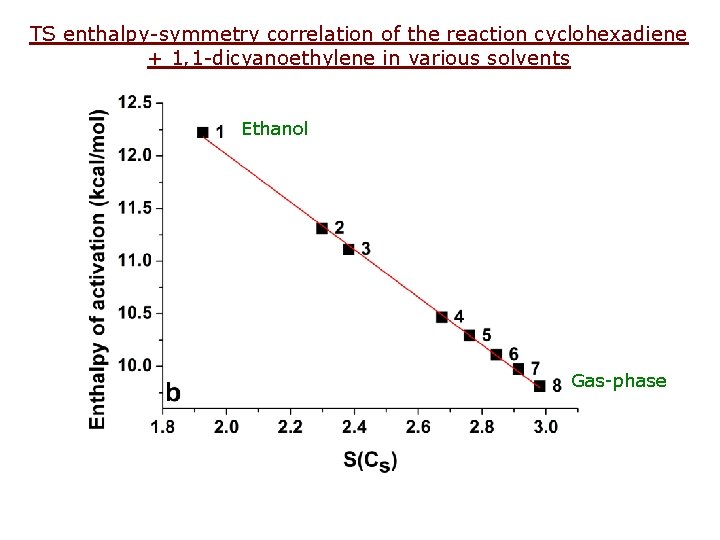
TS enthalpy-symmetry correlation of the reaction cyclohexadiene + 1, 1 -dicyanoethylene in various solvents Ethanol Gas-phase

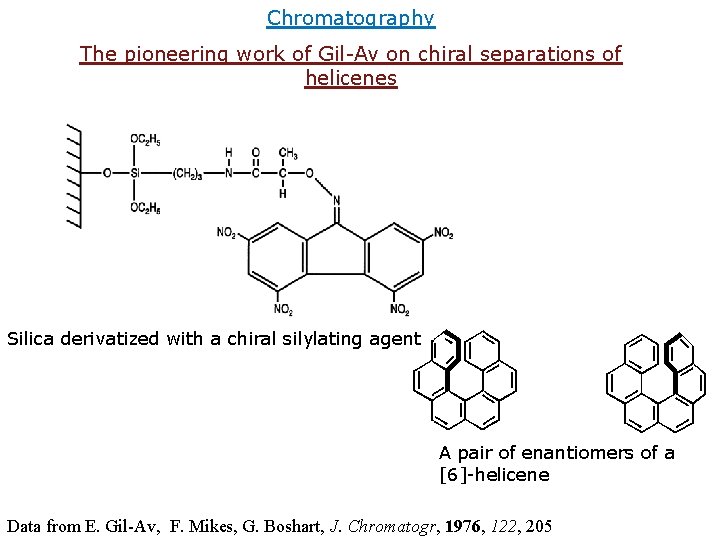
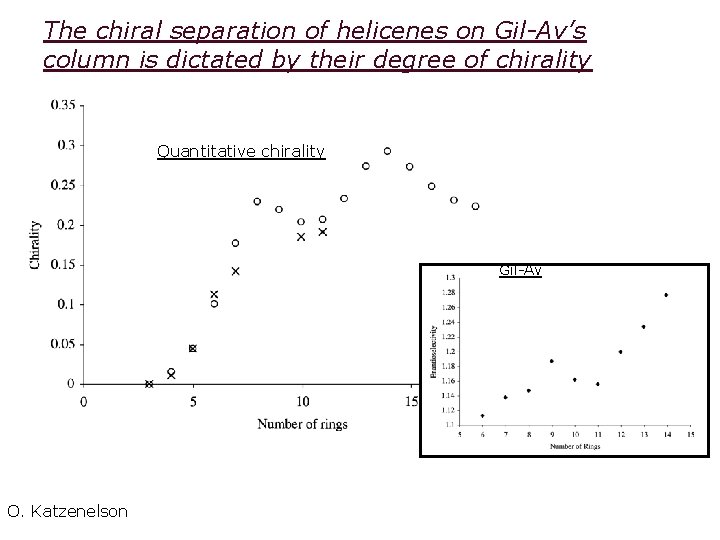
Chromatography The pioneering work of Gil-Av on chiral separations of helicenes Silica derivatized with a chiral silylating agent A pair of enantiomers of a [6]-helicene Data from E. Gil-Av, F. Mikes, G. Boshart, J. Chromatogr, 1976, 122, 205

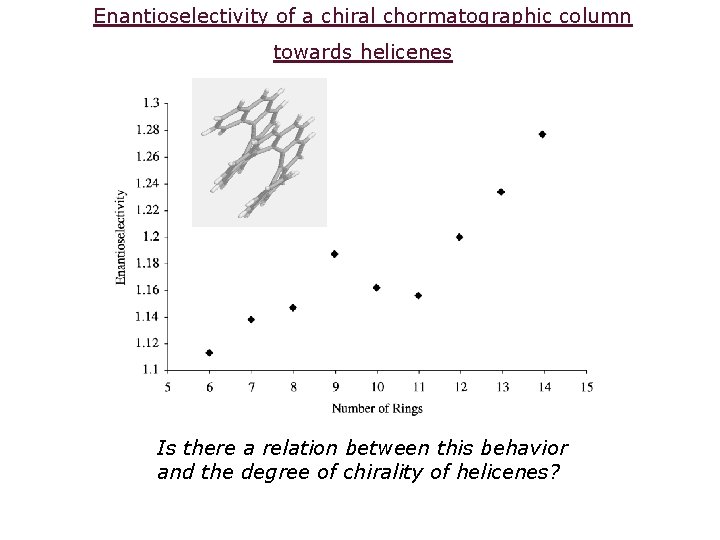
Enantioselectivity of a chiral chormatographic column towards helicenes Is there a relation between this behavior and the degree of chirality of helicenes?

The chiral separation of helicenes on Gil-Av’s column is dictated by their degree of chirality Quantitative chirality Gil-Av O. Katzenelson

6. Applications: Physics

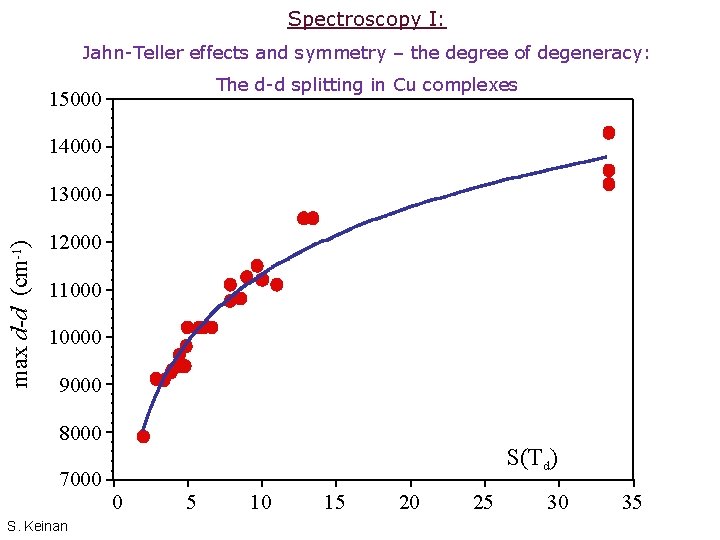
Spectroscopy I: Jahn-Teller effects and symmetry – the degree of degeneracy: The d-d splitting in Cu complexes 15000 14000 max d-d (cm-1) 13000 12000 110000 9000 8000 7000 S. Keinan S(Td) 0 5 10 15 20 25 30 35

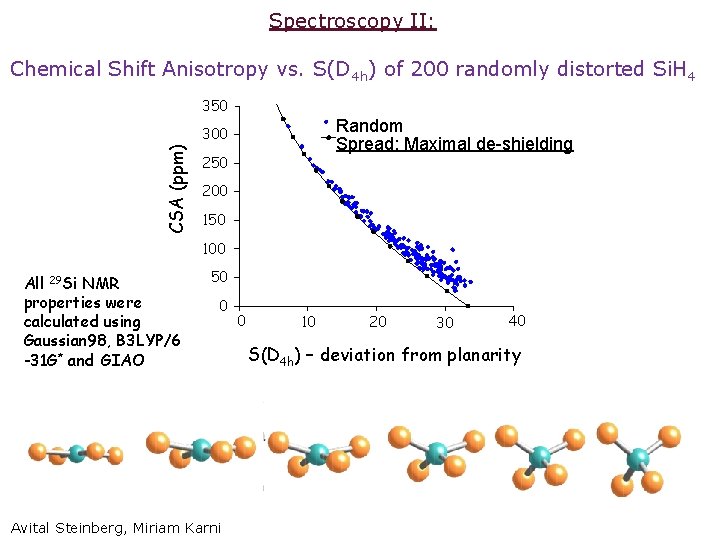
Spectroscopy II: Chemical Shift Anisotropy vs. S(D 4 h) of 200 randomly distorted Si. H 4 350 Random Spread: Maximal de-shielding CSA (ppm) 300 250 200 150 100 All 29 Si NMR properties were calculated using Gaussian 98, B 3 LYP/6 -31 G* and GIAO 50 0 Avital Steinberg, Miriam Karni 0 10 20 30 40 S(D 4 h) – deviation from planarity

Parity violation relations in metallic nanoclusters Parity violation energy differences The phenomenon - which originates in the chirality of the nuclear weak interaction - induces a tiny diasetereomeric energy difference between a pair of enantiomers, ∆EPV. It depends on the nuclear framework and on the number of nuclei Parity operation: Inversion of space Is there a correlation between the magnitude of the PV effect and the CCM value? Hadassah Elgavi ith Robert Berger and Christian Krekeler, Darmstadt

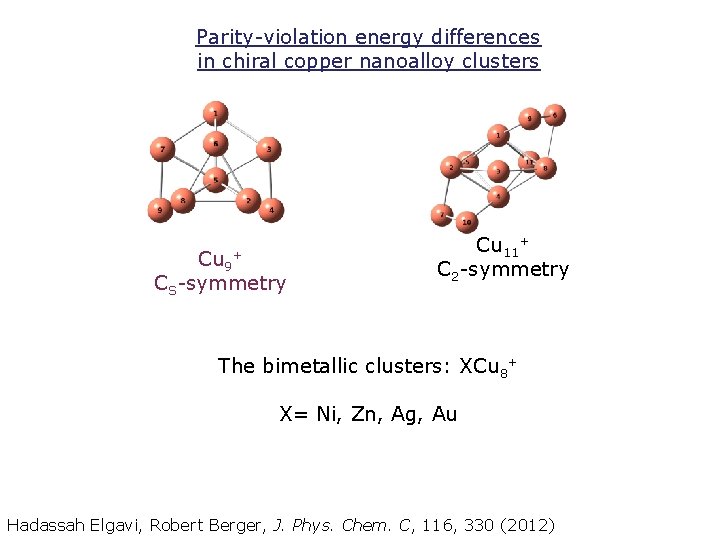
Parity-violation energy differences in chiral copper nanoalloy clusters Cu 9+ CS-symmetry Cu 11+ C 2 -symmetry The bimetallic clusters: XCu 8+ X= Ni, Zn, Ag, Au Hadassah Elgavi, Robert Berger, J. Phys. Chem. C, 116, 330 (2012)


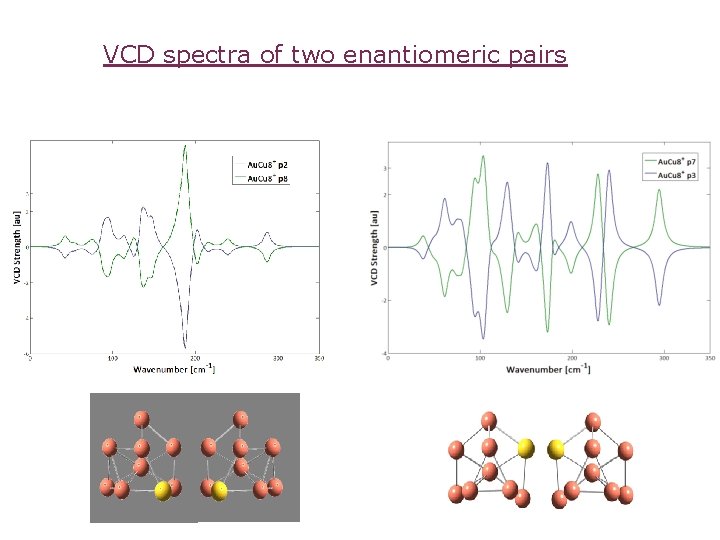
VCD spectra of two enantiomeric pairs

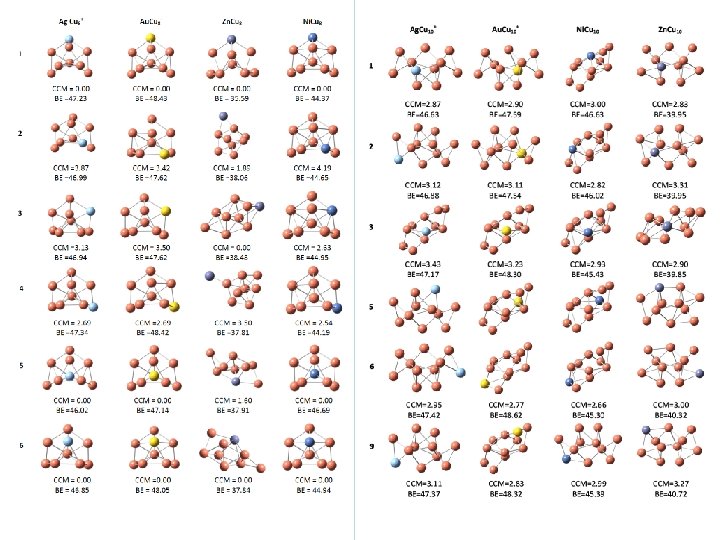
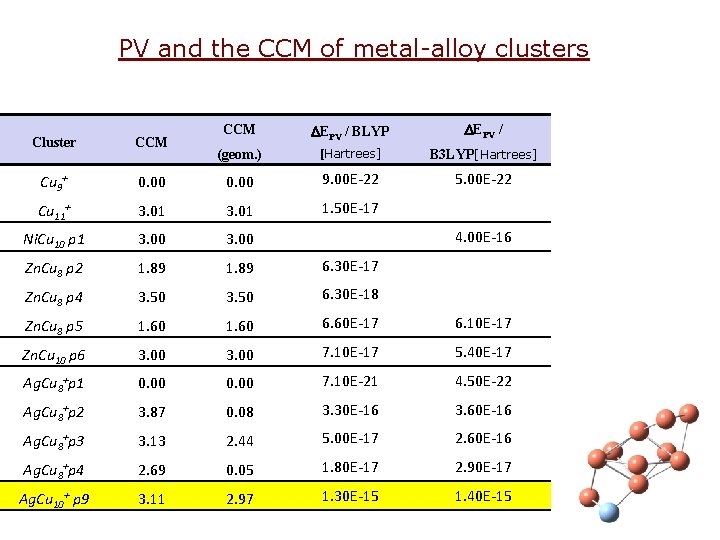
PV and the CCM of metal-alloy clusters CCM DEPV / BLYP DEPV / (geom. ) [Hartrees] B 3 LYP[Hartrees] 0. 00 9. 00 E-22 5. 00 E-22 Cu 11+ 3. 01 1. 50 E-17 Ni. Cu 10 p 1 3. 00 4. 00 E-16 Zn. Cu 8 p 2 1. 89 6. 30 E-17 Zn. Cu 8 p 4 3. 50 6. 30 E-18 Zn. Cu 8 p 5 1. 60 6. 60 E-17 6. 10 E-17 Zn. Cu 10 p 6 3. 00 7. 10 E-17 5. 40 E-17 Ag. Cu 8+p 1 0. 00 7. 10 E-21 4. 50 E-22 Ag. Cu 8+p 2 3. 87 0. 08 3. 30 E-16 3. 60 E-16 Ag. Cu 8+p 3 3. 13 2. 44 5. 00 E-17 2. 60 E-16 Ag. Cu 8+p 4 2. 69 0. 05 1. 80 E-17 2. 90 E-17 Ag. Cu 10+ p 9 3. 11 2. 97 1. 30 E-15 1. 40 E-15 Cluster CCM Cu 9+

7. Beyond the natural sciences

The Great Rift Valley

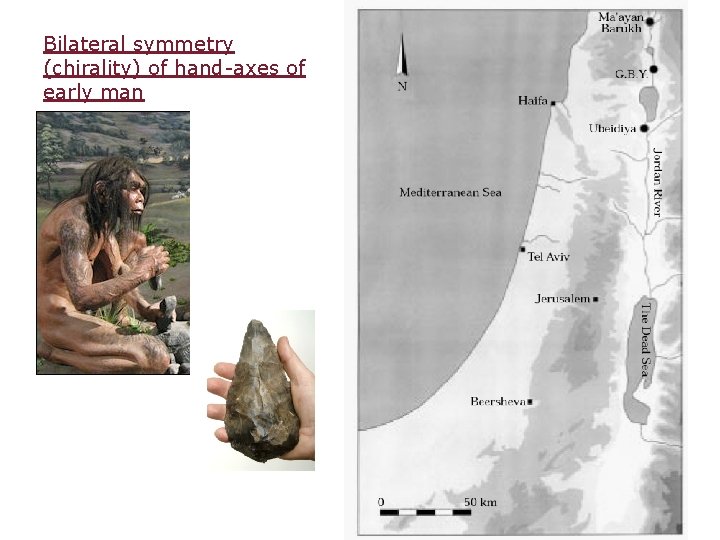
Bilateral symmetry (chirality) of hand-axes of early man

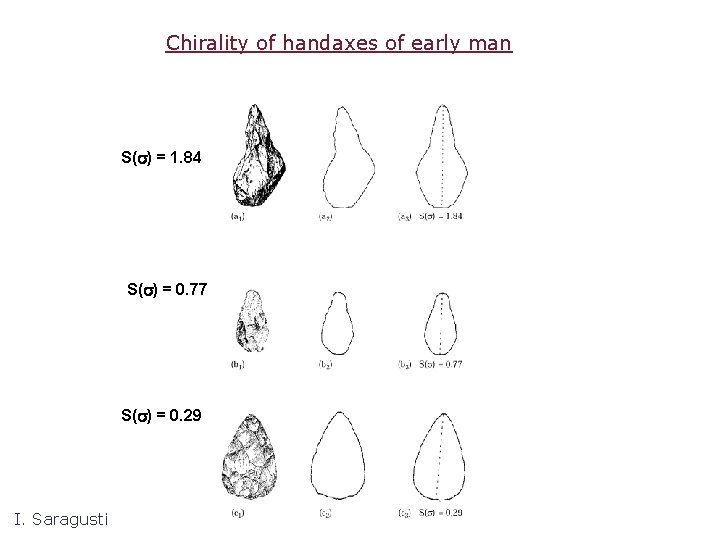
Chirality of handaxes of early man S(s) = 1. 84 S(s) = 0. 77 S(s) = 0. 29 I. Saragusti

Symmetry over 800000 years