CONTINUOUS IDEAL REACTORS A SARATH BABU 1 Continuous
CONTINUOUS IDEAL REACTORS A. SARATH BABU 1

Continuous Stirred Tank Reactor 2

CSTR Contd. . . 3

CSTR Animation 4
• Also called as Mixed, Backmix, Ideal stirred tank reactor • Open system, operates under steady state conditions • Reactants are continuously introduced and products are continuously withdrawn • Perfect mixing – contents have uniform properties – No spatial variations • Conditions at the exit are same as inside the reactor • Used for homogenous liquid phase reactions where constant agitation is required • Eg. Sulfonation, Polymerization, plastics, explosives, synthetic rubber etc. CSTR Contd. . . 5
Advantages: • Cheap to construct • Good temperature control • Reactor has large heat capacity • Easy access to interiors Disadvantages: • Conversion per unit volume of the reactor is smallest compared to other flow reactors CSTR Contd. . . 6
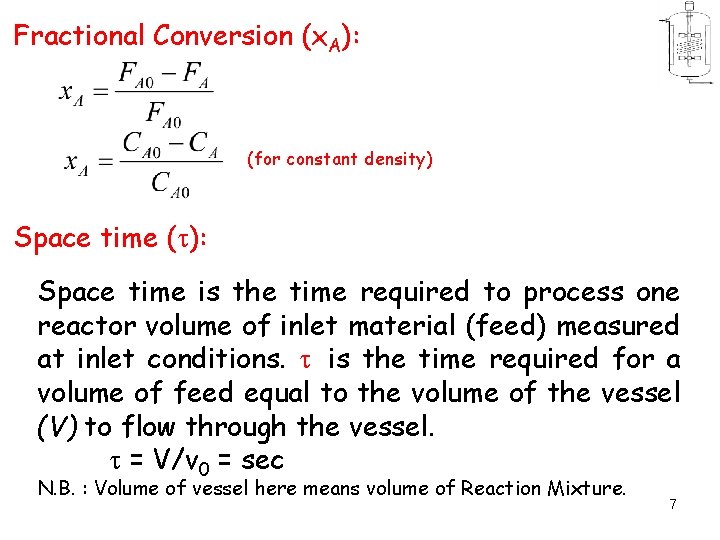
Fractional Conversion (x. A): (for constant density) Space time ( ): Space time is the time required to process one reactor volume of inlet material (feed) measured at inlet conditions. is the time required for a volume of feed equal to the volume of the vessel (V) to flow through the vessel. = V/v 0 = sec N. B. : Volume of vessel here means volume of Reaction Mixture. 7
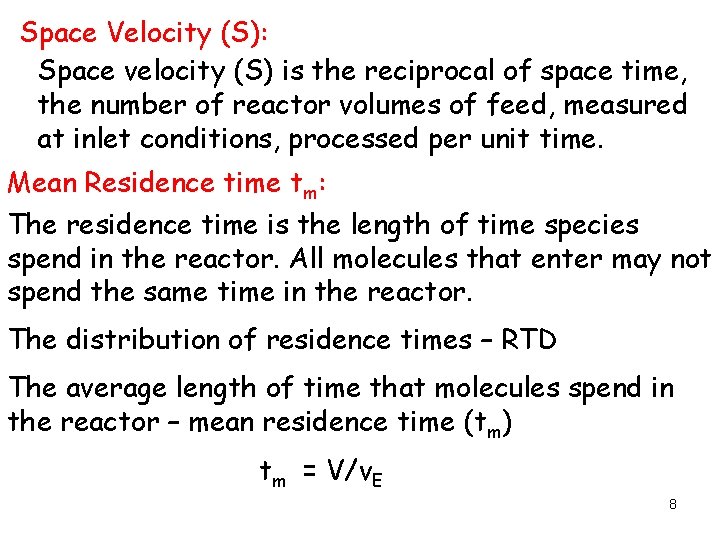
Space Velocity (S): Space velocity (S) is the reciprocal of space time, the number of reactor volumes of feed, measured at inlet conditions, processed per unit time. Mean Residence time tm: The residence time is the length of time species spend in the reactor. All molecules that enter may not spend the same time in the reactor. The distribution of residence times – RTD The average length of time that molecules spend in the reactor – mean residence time (tm) tm = V/v. E 8
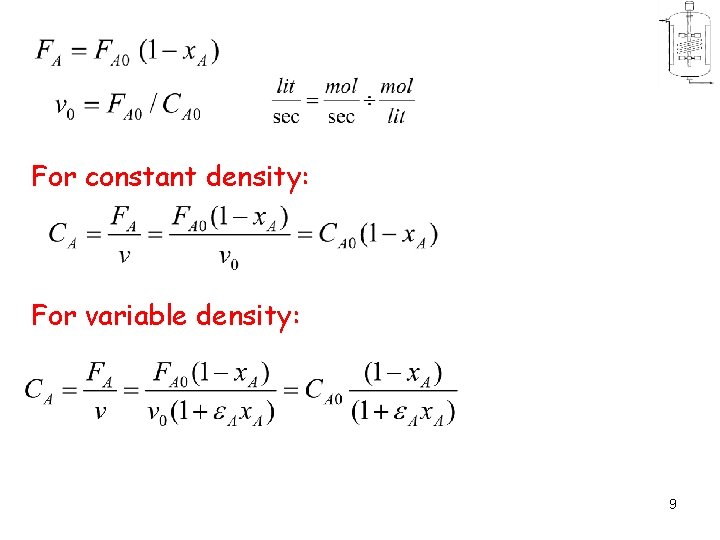
For constant density: For variable density: 9
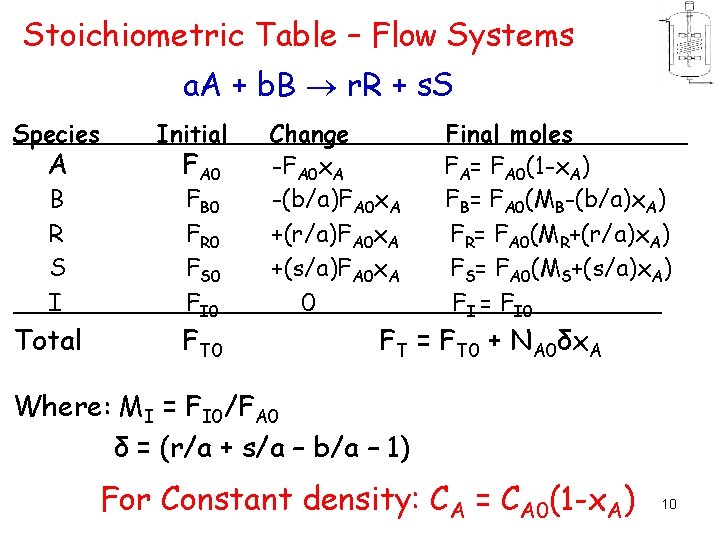
Stoichiometric Table – Flow Systems a. A + b. B r. R + s. S Species A B R S I Total Initial FA 0 FB 0 FR 0 FS 0 FI 0 FT 0 Change -FA 0 x. A -(b/a)FA 0 x. A +(r/a)FA 0 x. A +(s/a)FA 0 x. A 0 Final moles FA= FA 0(1 -x. A) FB= FA 0(MB-(b/a)x. A) FR= FA 0(MR+(r/a)x. A) FS= FA 0(MS+(s/a)x. A) FI = FI 0 FT = FT 0 + NA 0δx. A Where: MI = FI 0/FA 0 δ = (r/a + s/a – b/a – 1) For Constant density: CA = CA 0(1 -x. A) 10
11

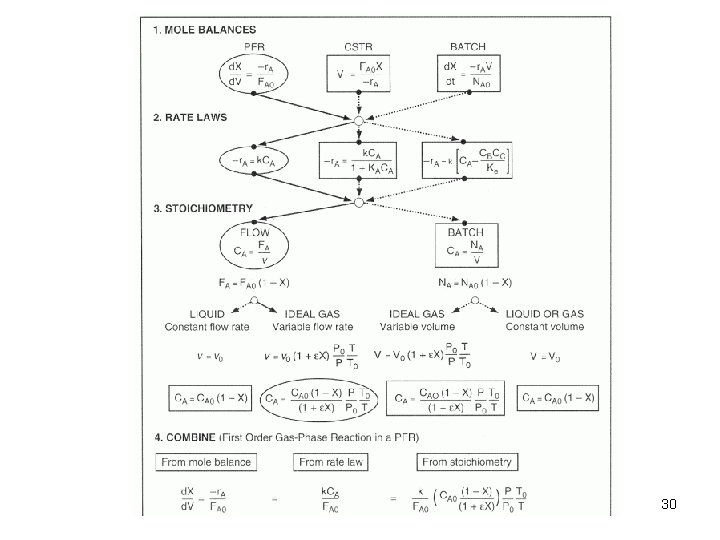
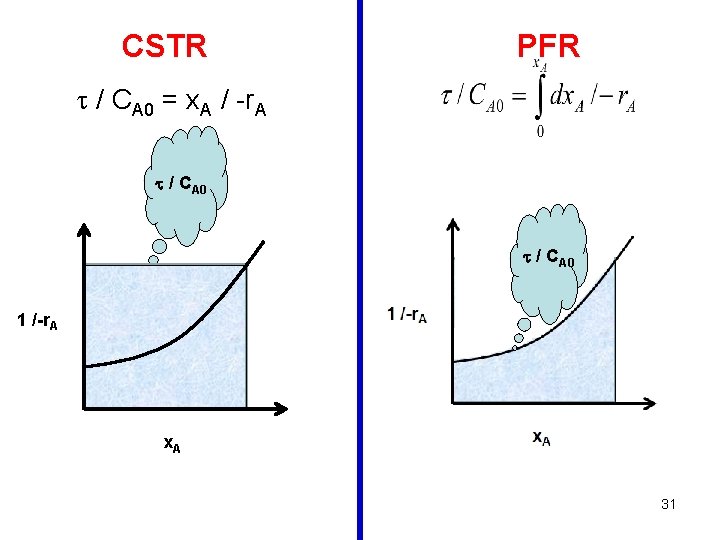
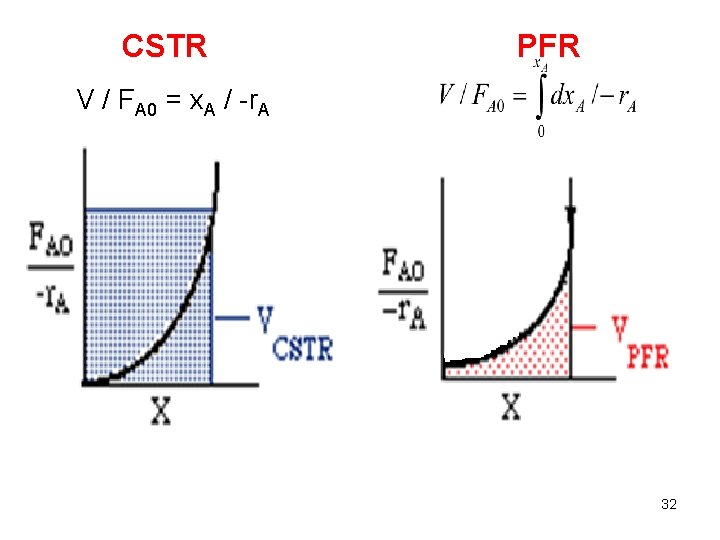
Design Equation General Mass Balance Equation: Rate of Input = rate of output + accumulation + rate of disappearance FA 0 = FA + 0 + (-r. A) V FA 0 - FA = (-r. A) V FA 0 CA 0 v 0 FA CA V x. A FA 0 x. A = (-r. A) V V / FAo = x. A / -r. A 12
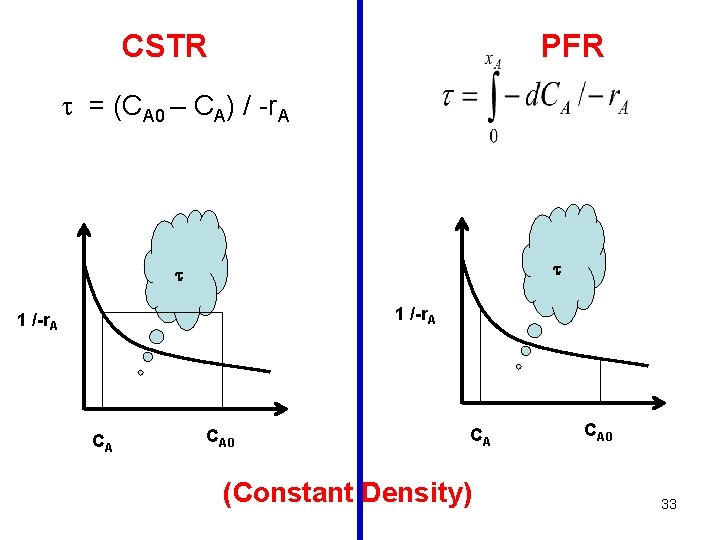
General Design eqn. for a CSTR: V / FAo = x. A / -r. A V / (v 0 CA 0) = x. A / -r. A / CA 0 = x. A / -r. A Design eqn. for a CSTR under constant density: = (CA 0 – CA) / -r. A tm = V/v. E Note that the space time and the mean residence time are equal only in the case of constant density. 13
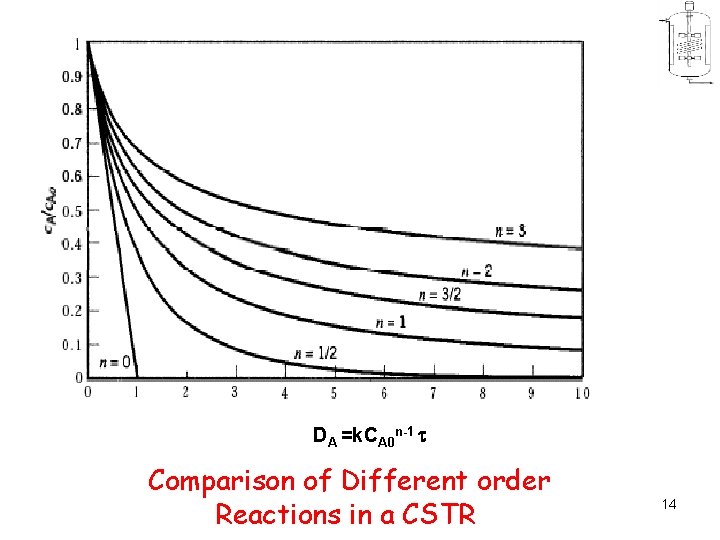
DA =k. CA 0 n-1 Comparison of Different order Reactions in a CSTR 14
Plug Flow Reactor 15
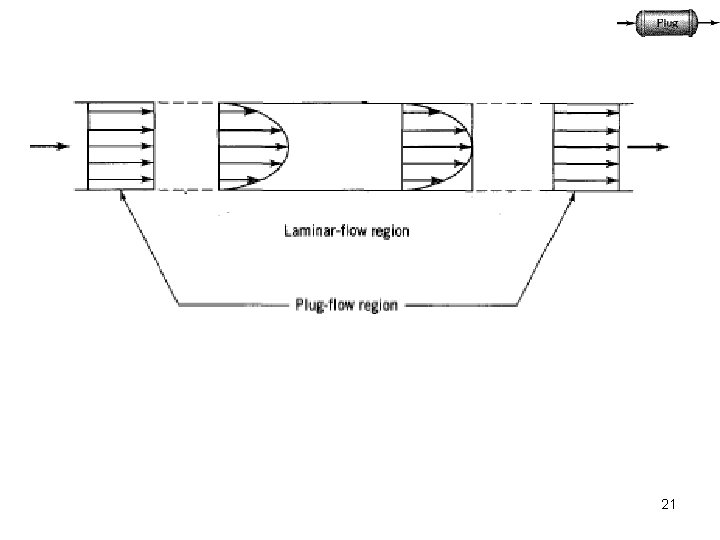
The necessary and sufficient condition for plug flow is the residence time in the reactor to be the same for all elements of the fluid. PFR Animation 16
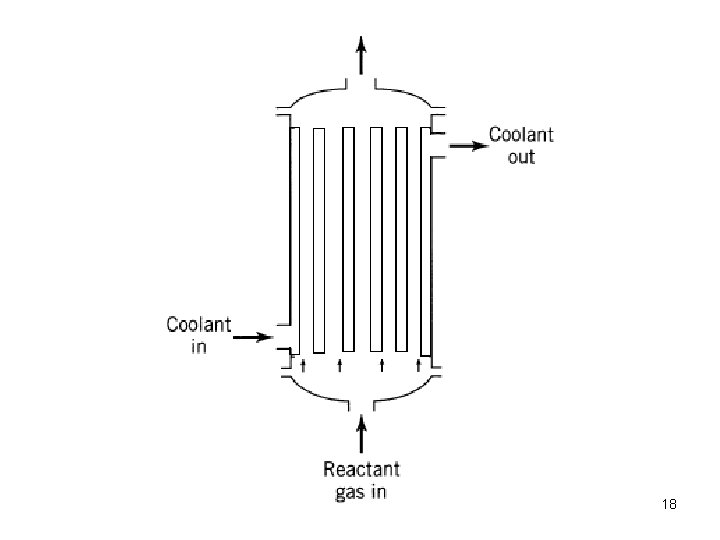
• PFR is also called as tubular reactor • Residence time is same for all fluid elements • Operated under steady state conditions • Reactants are consumed as they flow down along the length of the reactor • Axial concentration gradients exist • One long tube or a number of short tubes (see fig. ) • Choice of diameter depends on fabrication cost, pumping cost and heat transfer needs • Wide variety of applications in gas/liquid phase • Eg. : Production of gasoline, cracking, synthesis of ammonia, SO 2 oxidation 17
18

(1) The flow in the vessel is Plug flow. (2)There is no axial mixing of fluid inside the vessel (i. e. , in the direction of flow). (3)There is complete radial mixing of fluid inside the vessel (i. e. , in the plane perpendicular to the direction of flow). (4)Properties may change continuously in the direction of flow (5)In the axial direction, each portion of fluid, acts as a closed system in motion, not exchanging material with the portion ahead of it or behind it. 19
Advantages: • Easily maintained as there are no moving parts • High conversion per unit volume • Unvarying product quality • Good for studying rapid reactions Disadvantages: • Poor temperature control • Hot spots may occur when used for exothermic reactions PFR Contd. . . 20
21
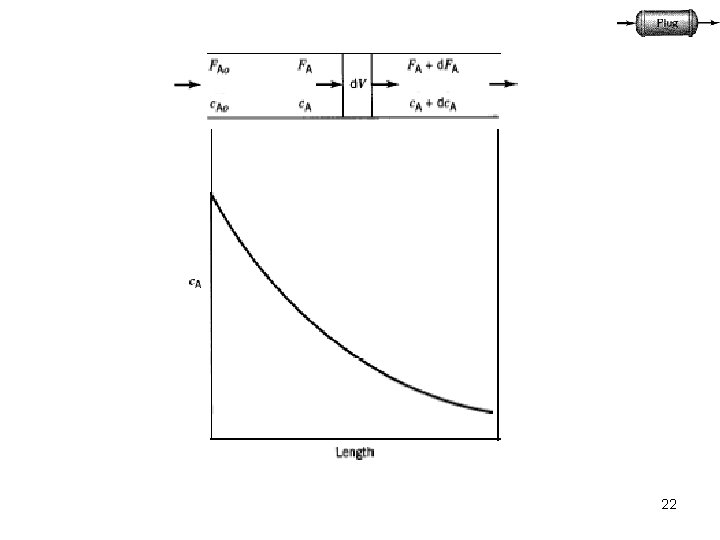
22
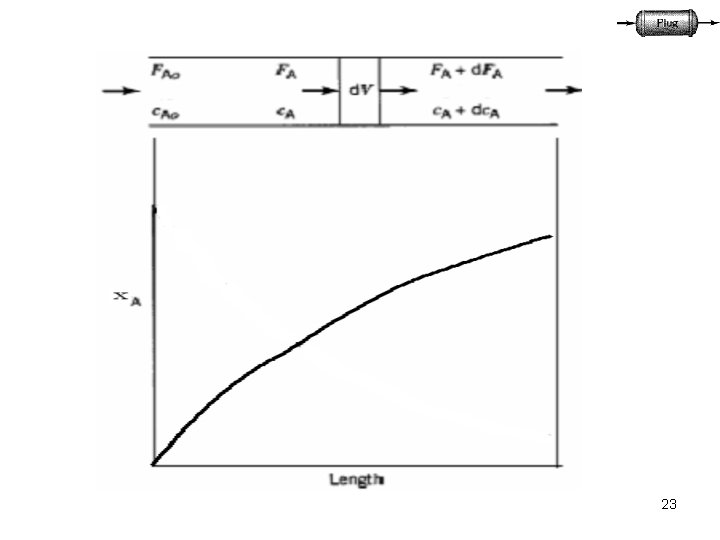
23
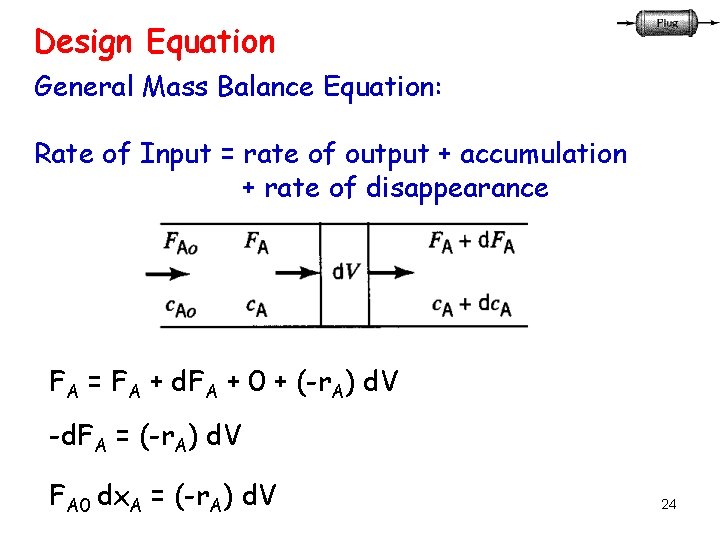
Design Equation General Mass Balance Equation: Rate of Input = rate of output + accumulation + rate of disappearance FA = FA + d. FA + 0 + (-r. A) d. V -d. FA = (-r. A) d. V FA 0 dx. A = (-r. A) d. V 24
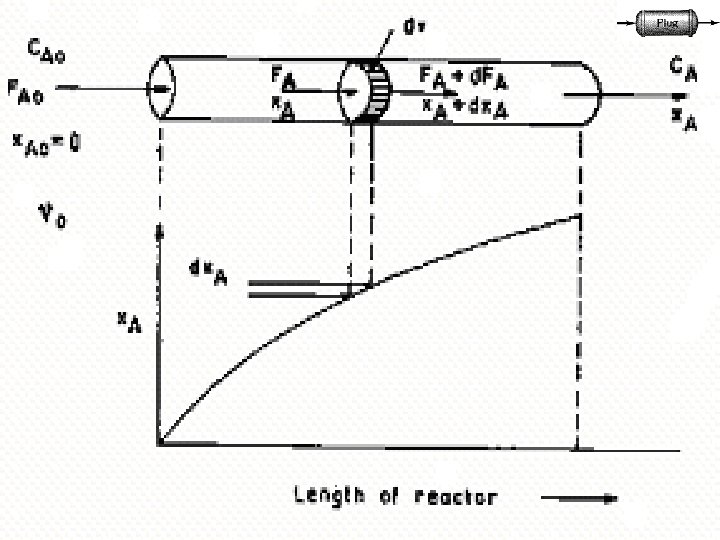
25
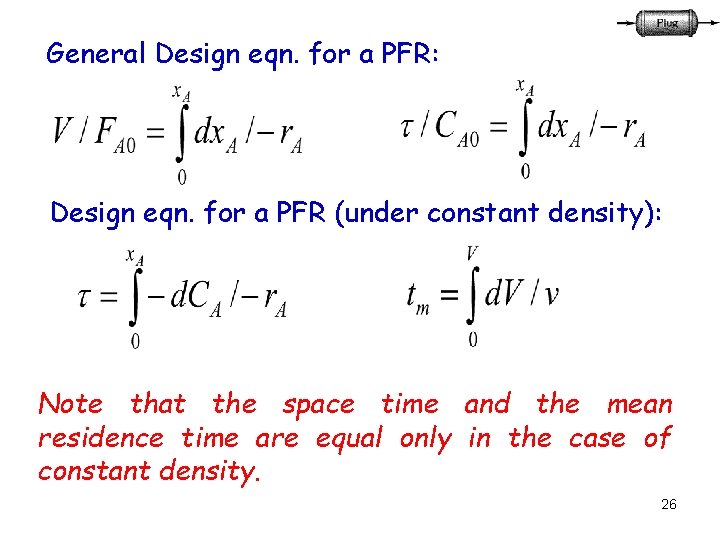
General Design eqn. for a PFR: Design eqn. for a PFR (under constant density): Note that the space time and the mean residence time are equal only in the case of constant density. 26
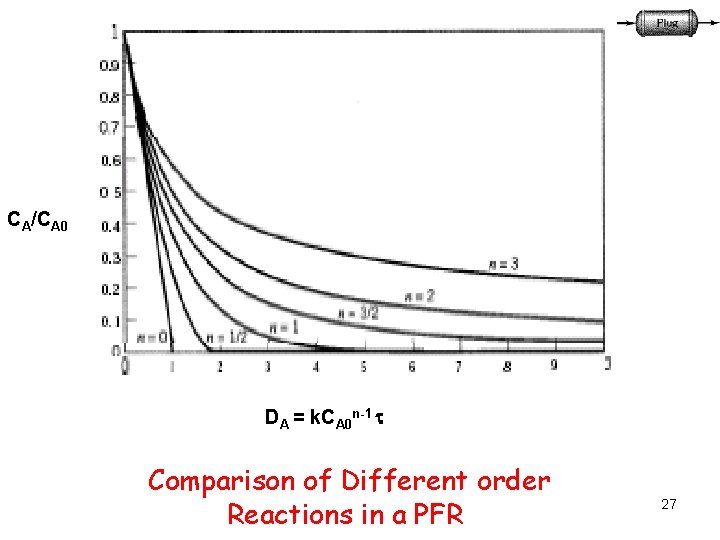
CA/CA 0 DA = k. CA 0 n-1 Comparison of Different order Reactions in a PFR 27
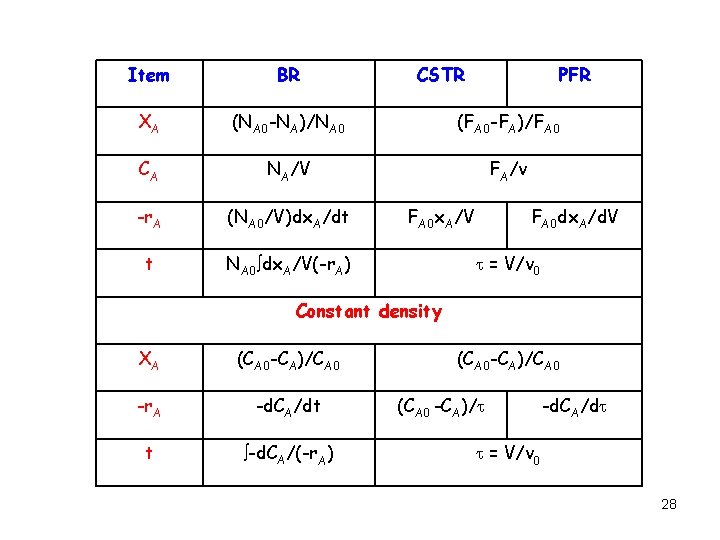
Item BR CSTR PFR XA (NA 0 -NA)/NA 0 (FA 0 -FA)/FA 0 CA NA/V FA/v -r. A (NA 0/V)dx. A/dt t NA 0 dx. A/V(-r. A) FA 0 x. A/V FA 0 dx. A/d. V = V/v 0 Constant density XA (CA 0 -CA)/CA 0 -r. A -d. CA/dt t -d. CA/(-r. A) (CA 0 -CA)/CA 0 (CA 0 -CA)/ -d. CA/d = V/v 0 28
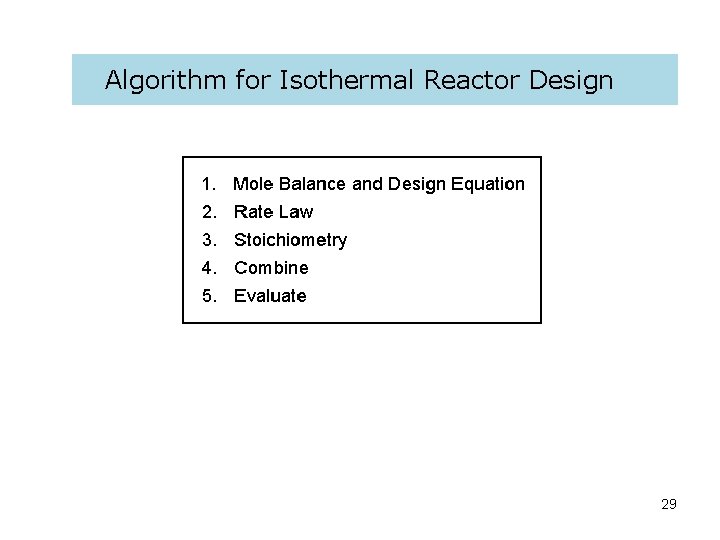
Algorithm for Isothermal Reactor Design 29
30
CSTR PFR / CA 0 = x. A / -r. A / CA 0 1 /-r. A x. A 31
CSTR PFR V / FA 0 = x. A / -r. A 32
CSTR PFR = (CA 0 – CA) / -r. A 1 /-r. A CA CA 0 CA (Constant Density) CA 0 33
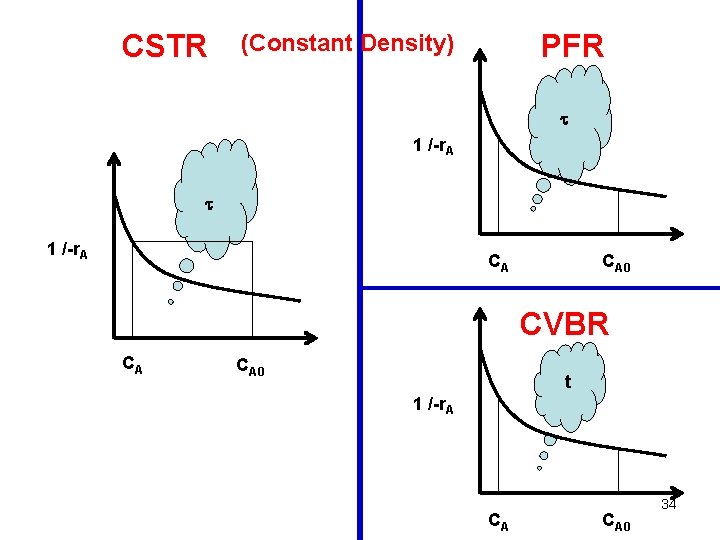
CSTR PFR (Constant Density) 1 /-r. A CA CA 0 CVBR CA CA 0 t 1 /-r. A CA CA 0 34
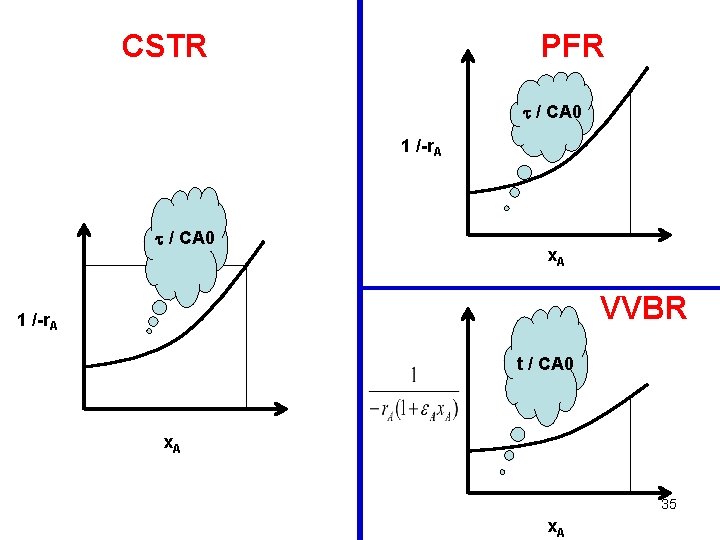
CSTR PFR / CA 0 1 /-r. A / CA 0 x. A VVBR 1 /-r. A t / CA 0 x. A 35 x. A
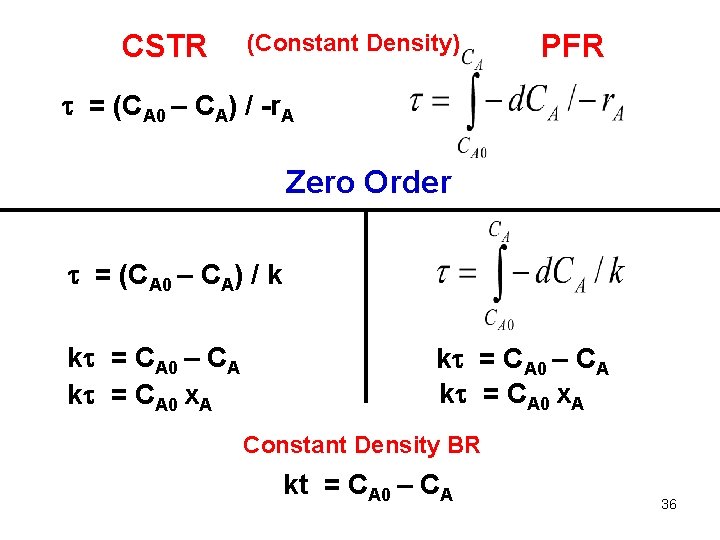
CSTR (Constant Density) PFR = (CA 0 – CA) / -r. A Zero Order = (CA 0 – CA) / k k = CA 0 – CA k = CA 0 x. A Constant Density BR kt = CA 0 – CA 36
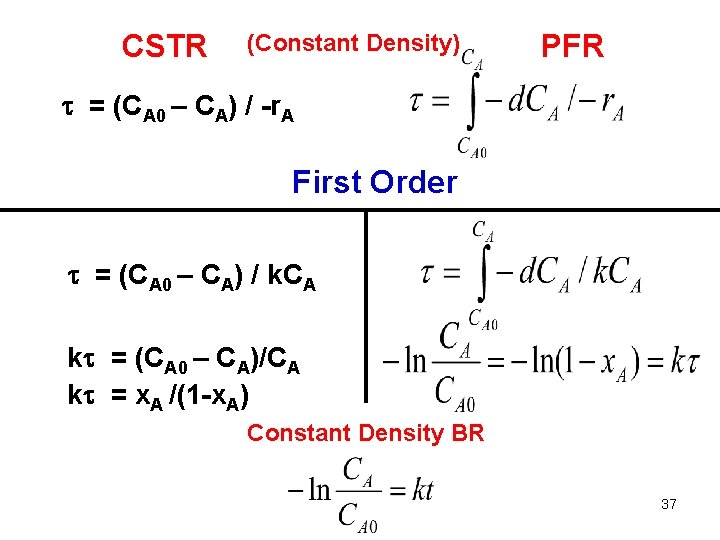
CSTR (Constant Density) PFR = (CA 0 – CA) / -r. A First Order = (CA 0 – CA) / k. CA k = (CA 0 – CA)/CA k = x. A /(1 -x. A) Constant Density BR 37
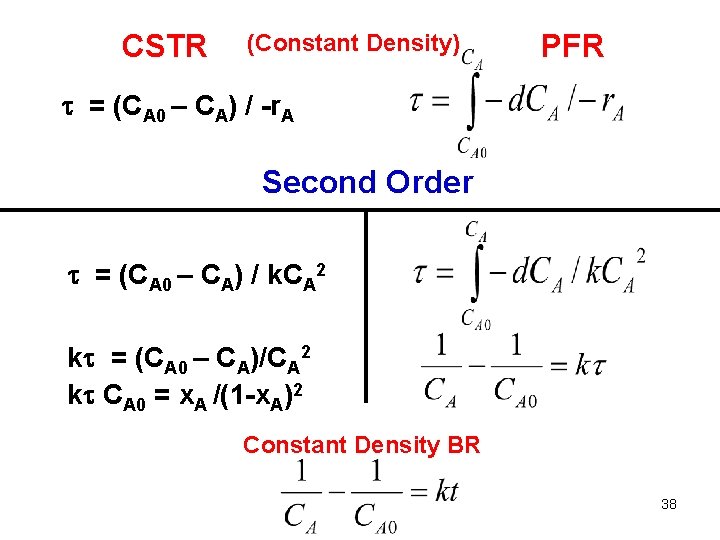
CSTR (Constant Density) PFR = (CA 0 – CA) / -r. A Second Order = (CA 0 – CA) / k. CA 2 k = (CA 0 – CA)/CA 2 k CA 0 = x. A /(1 -x. A)2 Constant Density BR 38
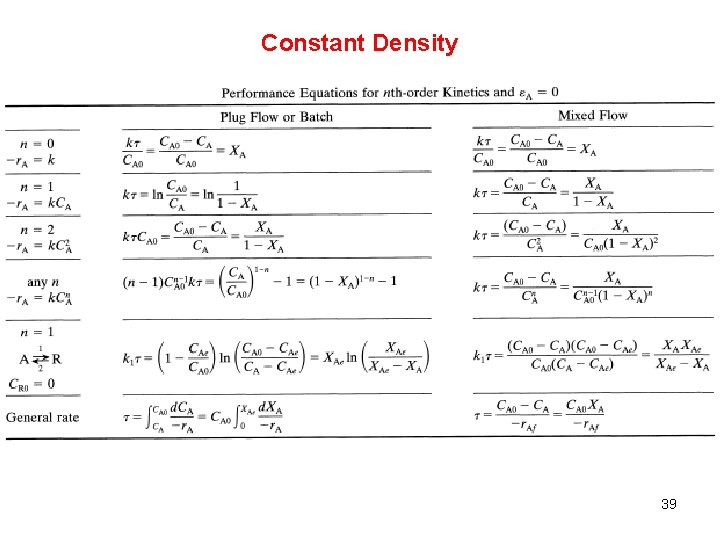
Constant Density 39

For constant density: • The performance of the Batch reactor is similar to that of PFR for all orders • The performance of all the three reactors is the same in case of zero order reaction • The performance of PFR is superior to that of a CSTR for all orders > 0 For all reaction orders > 0 • The volume of a CSTR required for obtaining a given conversion is larger than that of PFR • For the same volumes of PFR & CSTR, the conversion obtained is larger in the case of PFR 40
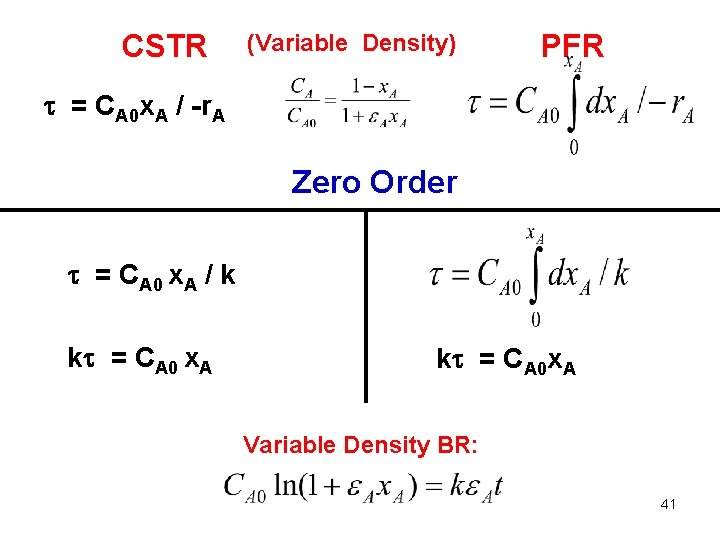
CSTR (Variable Density) PFR = CA 0 x. A / -r. A Zero Order = CA 0 x. A / k k = CA 0 x. A k = CA 0 x. A Variable Density BR: 41
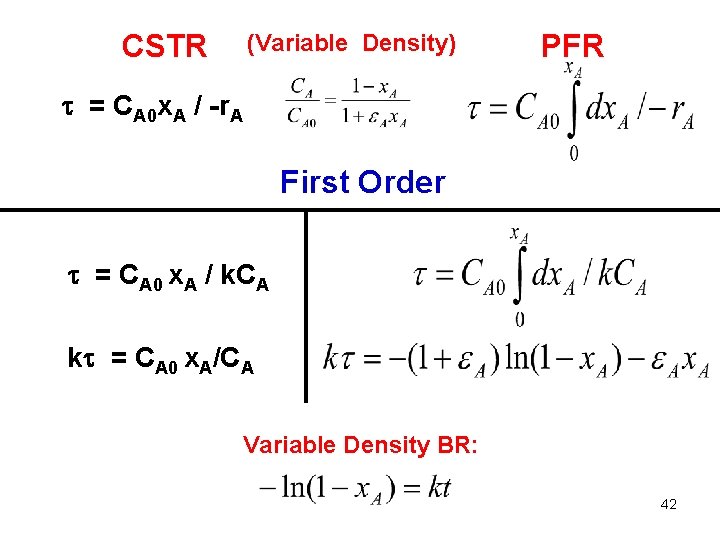
CSTR (Variable Density) PFR = CA 0 x. A / -r. A First Order = CA 0 x. A / k. CA k = CA 0 x. A/CA Variable Density BR: 42
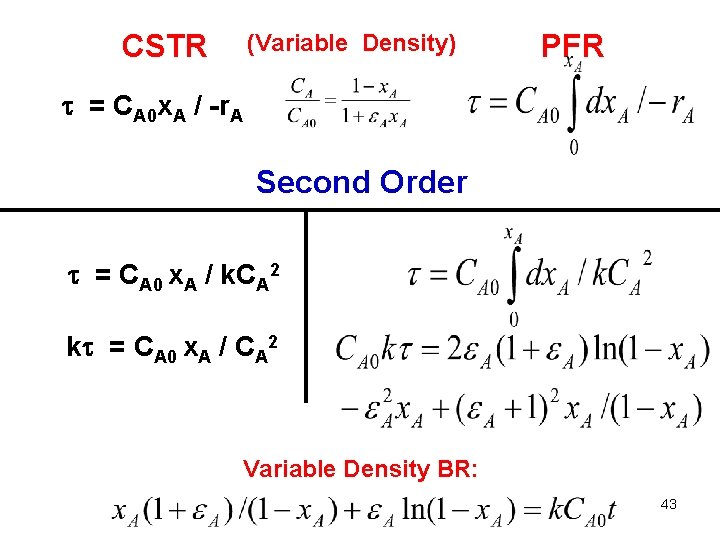
CSTR (Variable Density) PFR = CA 0 x. A / -r. A Second Order = CA 0 x. A / k. CA 2 k = CA 0 x. A / CA 2 Variable Density BR: 43
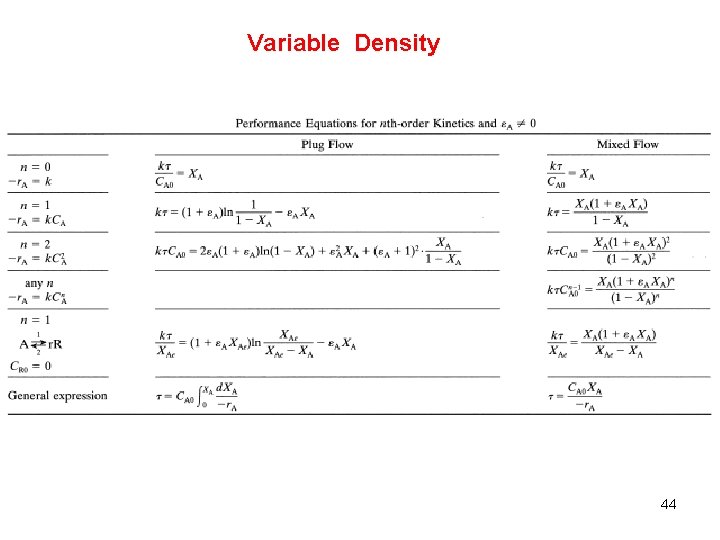
Variable Density 44
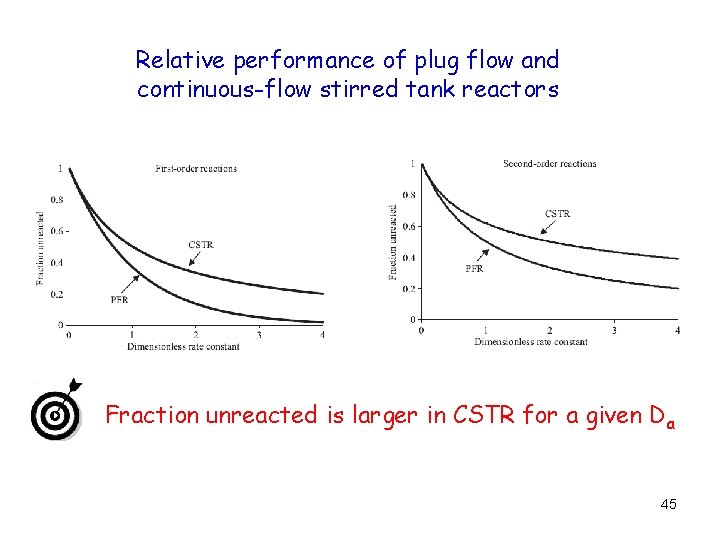
Relative performance of plug flow and continuous-flow stirred tank reactors Fraction unreacted is larger in CSTR for a given D a 45
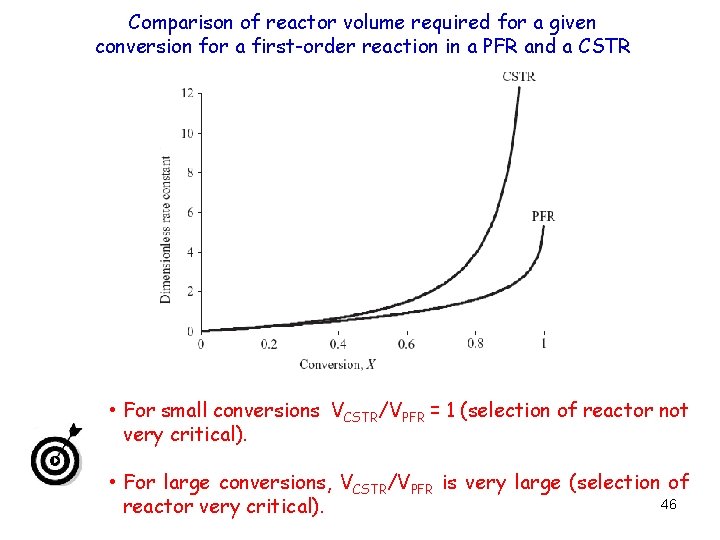
Comparison of reactor volume required for a given conversion for a first-order reaction in a PFR and a CSTR • For small conversions VCSTR/VPFR = 1 (selection of reactor not very critical). • For large conversions, VCSTR/VPFR is very large (selection of 46 reactor very critical).
For Variable density: • The performance of CSTR & PFR is similar in case of zero order (irrespective of constant / variable density) • The performance of BR is different from the performance of PFR (the performance was similar in the case of constant density) • The performance of PFR is superior to that of a CSTR for all orders > 0 (same as constant density) 47
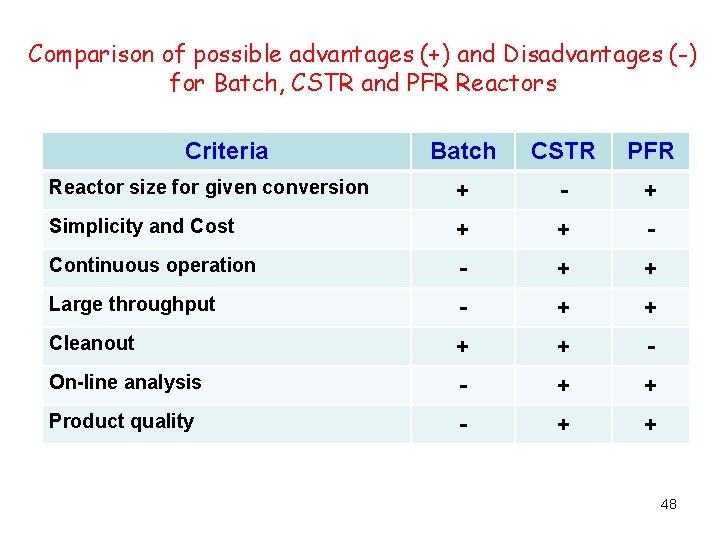
Comparison of possible advantages (+) and Disadvantages (-) for Batch, CSTR and PFR Reactors Criteria Batch CSTR PFR Reactor size for given conversion + - + Simplicity and Cost + + - Continuous operation - + + Large throughput - + + Cleanout + + - On-line analysis - + + Product quality - + + 48

ANY CLARIFICATIONS ? Abbey, Edward That which today calls itself science gives us more and more information, an indigestible glut of information, and less understanding. 49
- Slides: 49