Continued THE PERIODIC TABLE Structure of the Periodic
Continued. . . THE PERIODIC TABLE
Structure of the Periodic Table � Each element is listed according to its atomic number row by row from left to right. � Metals are on the LEFT and in the MIDDLE. � Non-metals are in the upper RIGHT corner � Metalloids form a staircase towards the right side.
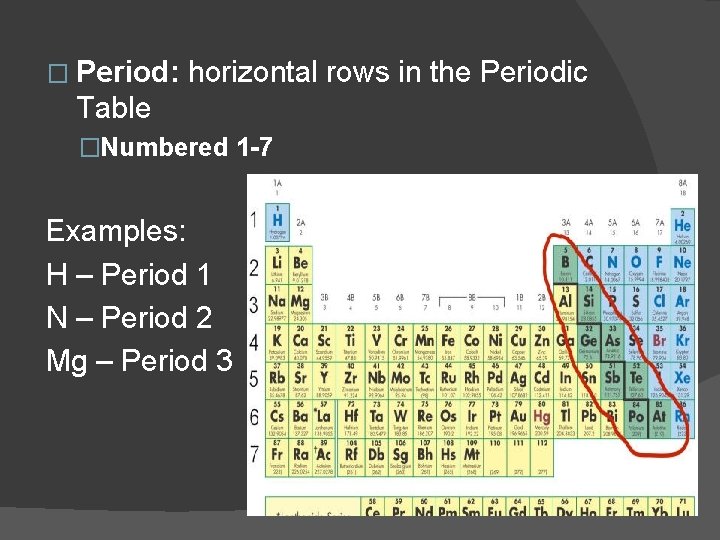
� Period: horizontal rows in the Periodic Table �Numbered 1 -7 Examples: H – Period 1 N – Period 2 Mg – Period 3

The Four Families No, I’m not talking about the Mafia. I’m talking about the 5 families on the Periodic Table
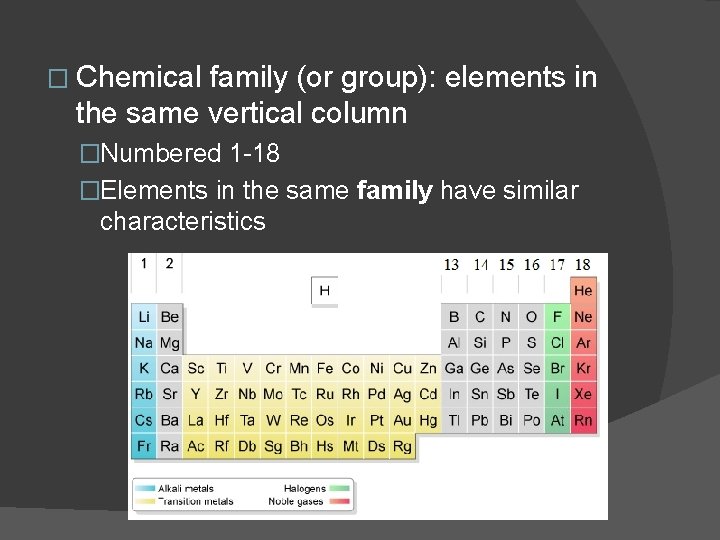
� Chemical family (or group): elements in the same vertical column �Numbered 1 -18 �Elements in the same family have similar characteristics

Alkali Metals � Group 1 (excluding hydrogen) � HIGHLY reactive �Reactivity increases as you move down � React with oxygen and water � Low melting points (below 200ºC) � Soft (can be cut with a knife) �Cesium is softer/more reactive than lithium


Alkaline Earth Metals � Group 2 � Less reactive than alkali metals, BUT will burn in the air if heated � Produce bright flames (used in fireworks) � React with water (not as vigorously as alkali metals) � Calcium reacts more quickly than magnesium


Halogens � � Group 17 Non-metals Highly reactive At room temperature. . . �Gas – Fluorine and Chlorine �Liquid – Bromine �Solid – Iodine Fluorine is the most reactive, Iodine is the least reactive � Astatine is so rare that no one has EVER collected enough to determine its physical properties �


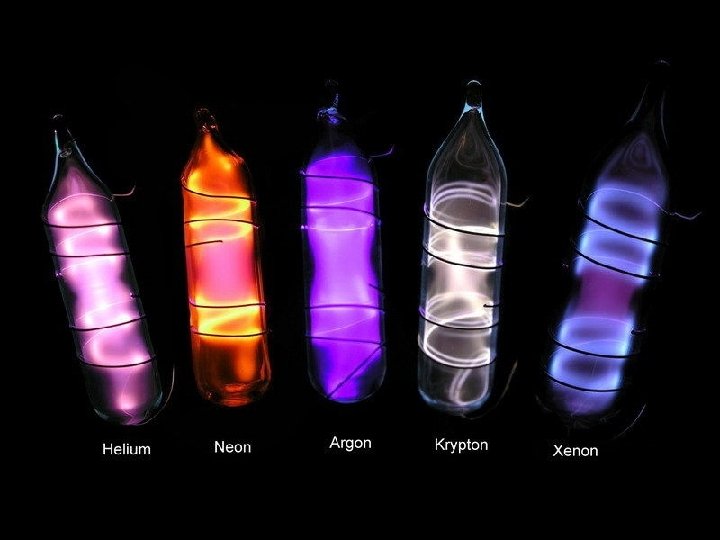
Noble Gases � Group 18 � The most stable and unreactive elements � At room temperature. . . �Colourless, odourless gases � Some are used in light fixtures (Argon and Neon) � Some glow in distinctive colours (Neon) � Helium is lighter than air
- Slides: 13