Content ICH Q 9 QUALITY RISK MANAGEMENT Quality






















- Slides: 48

Content ICH Q 9 QUALITY RISK MANAGEMENT Quality Risk Management ICH Q 9 Content Disclaimer: This presentation includes the authors views on quality risk management theory and practice. The presentation does not represent official guidance or policy of authorities or industry. prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 1

Content ICH Q 9 QUALITY RISK MANAGEMENT Purpose of this part · To guide through the content of the ICH Q 9 document · Provide some considerations, possible interpretations and where appropriate examples prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 2

Content ICH Q 9 QUALITY RISK MANAGEMENT ICH Q 9: Quality Risk Management (QRM) · Document is available on the ICH Webpage www. ich. org prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 3

Content ICH Q 9 QUALITY RISK MANAGEMENT Table of contents 1. Introduction 2. Scope 3. Principles of Quality Risk Management 4. General Quality Risk Management Process 5. Risk Management Methodology Annex I: Risk Management Methods and Tools 6. Integration of QRM process into Industry and Regulatory operations Annex II: Potential Applications for QRM 7. Definitions 8. References prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 4

Content ICH Q 9 QUALITY RISK MANAGEMENT 1. Introduction Risk Management Quality Systems Harm Severity Stakeholder Product Life Cycle GMP Compliance prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 5

Content ICH Q 9 QUALITY RISK MANAGEMENT 2. Scope This guideline provides principles & examples of tools of quality risk management that can be applied to different aspects of pharmaceutical quality. These aspects include development, manufacturing, distribution, and the inspection and submission/review processes throughout the lifecycle of drug substances, drug (medicinal) products, biological and biotechnological products ICH Q 9 prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 6

Content ICH Q 9 QUALITY RISK MANAGEMENT CONSIDERATIONS 2. Scope · Drug substances, · Drug (medicinal) products, · Biological and biotechnological products Including the selection and use of > Raw materials > Solvents > Excipients > Packaging and labelling materials > Components prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 7

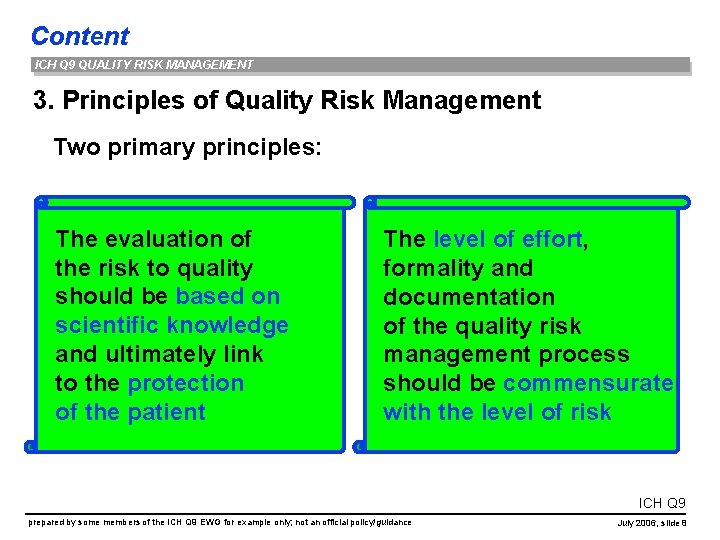
Content ICH Q 9 QUALITY RISK MANAGEMENT 3. Principles of Quality Risk Management Two primary principles: The evaluation of the risk to quality should be based on scientific knowledge and ultimately link to the protection of the patient The level of effort, formality and documentation of the quality risk management process should be commensurate with the level of risk ICH Q 9 prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 8

Content ICH Q 9 QUALITY RISK MANAGEMENT 4. General Quality Risk Management Process Systematic processes designed to coordinate, facilitate and improve science-based decision making with respect to risk to quality ICH Q 9 prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 9

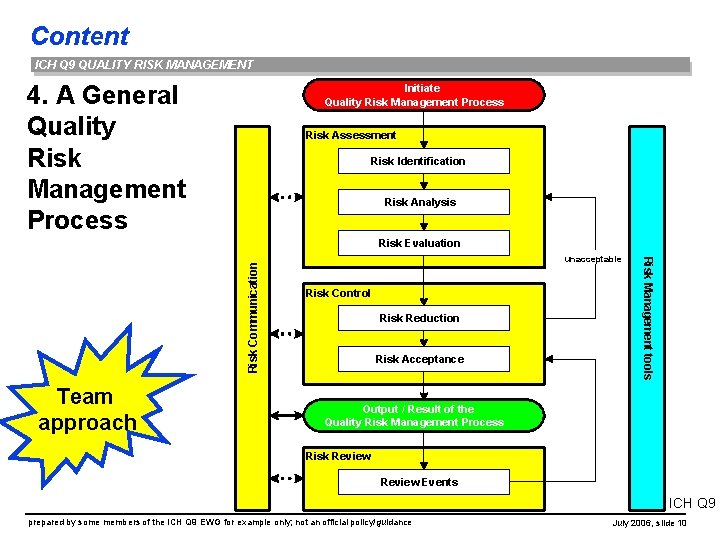
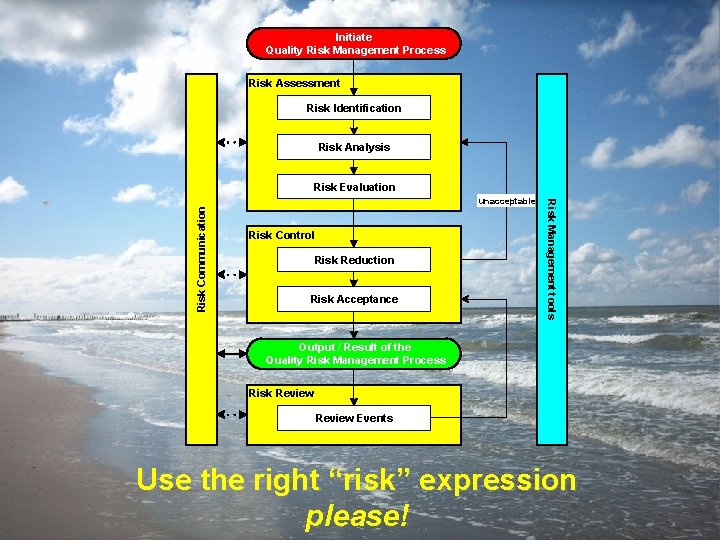
Content ICH Q 9 QUALITY RISK MANAGEMENT 4. A General Quality Risk Management Process Initiate Quality Risk Management Process Risk Assessment Risk Identification Risk Analysis Team approach unacceptable Risk Control Risk Reduction Risk Acceptance Risk Management tools Risk Communication Risk Evaluation Output / Result of the Quality Risk Management Process Risk Review Events ICH Q 9 prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 10

Content ICH Q 9 QUALITY RISK MANAGEMENT 4. General Quality Risk Management Process Decision makers: Person(s) with competence and authority to make a decision · Coordinating quality risk management process across various functions and departments ICH Q 9 · Supporting the team approach prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance Management responsibility · Ensuring that ongoing Quality Risk Management processes operate July 2006, slide 11

Content ICH Q 9 QUALITY RISK MANAGEMENT CONSIDERATIONS 4. General Quality Risk Management Process Team approach · Usually, but not always, undertaken by interdisciplinary teams from areas appropriate to the risk being considered e. g. > > > > > Quality unit Development Engineering / Statistics Regulatory affairs Production operations Business, Sales and Marketing Legal Medical / Clinical &… Individuals knowledgeable of the QRM processes prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 12

Content ICH Q 9 QUALITY RISK MANAGEMENT 4. General Quality Risk Management Process When to initiate and plan a QRM Process · First define the question which should be answered (e. g. a problem and/or risk question) > including pertinent assumptions identifying the potential for risk · Then assemble background information and/ or data on the potential hazard, harm or human health impact relevant to the risk > Identify a leader and necessary resources Initiate Quality Risk Management Process Risk Assessment prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance Risk Analysis Risk Communication Risk Evaluation unacceptable Risk Control Risk Reduction Risk Acceptance Output / Result of the Quality Risk Management Process ICH Q 9 Risk Management tools > Specify a timeline, deliverables and appropriate level of decision making for the QRM process Risk Identification Risk Review Events July 2006, slide 13

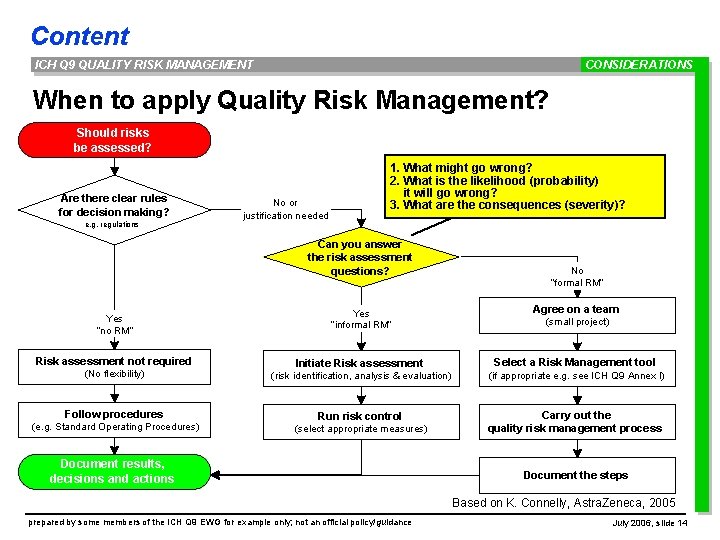
Content ICH Q 9 QUALITY RISK MANAGEMENT CONSIDERATIONS When to apply Quality Risk Management? Should risks be assessed? Are there clear rules for decision making? e. g. regulations No or justification needed 1. What might go wrong? 2. What is the likelihood (probability) it will go wrong? 3. What are the consequences (severity)? Can you answer the risk assessment questions? Yes “no RM“ Risk assessment not required (No flexibility) Follow procedures (e. g. Standard Operating Procedures) Yes “informal RM“ No “formal RM“ Agree on a team (small project) Initiate Risk assessment Select a Risk Management tool (risk identification, analysis & evaluation) (if appropriate e. g. see ICH Q 9 Annex I) Run risk control Carry out the quality risk management process (select appropriate measures) Document results, decisions and actions Document the steps Based on K. Connelly, Astra. Zeneca, 2005 prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 14

Content ICH Q 9 QUALITY RISK MANAGEMENT 4. General Quality Risk Management Process Risk Assessment · Risk Identification What might go wrong? 3 fundamental questions · Risk Analysis What is the likelihood (probability) it will go wrong? · Risk Evaluation What are the consequences (severity)? Note: People often use terms “Risk analysis”, “Risk assessment” and “Risk management” interchangeably which is incorrect! Initiate Quality Risk Management Process Risk Assessment Risk Identification Risk Analysis prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance Risk Communication unacceptable Risk Control Risk Reduction Risk Acceptance Output / Result of the Quality Risk Management Process ICH Q 9 Risk Management tools Risk Evaluation Risk Review Events July 2006, slide 15

Content ICH Q 9 QUALITY RISK MANAGEMENT 4. General Quality Risk Management Process Risk Assessment: Risk Identification “What might go wrong? ” · A systematic use of information to identify hazards referring to the risk question or problem > historical data > theoretical analysis Initiate Quality Risk Management Process > informed opinions Risk Assessment Risk Identification Risk Analysis Risk Communication > concerns of stakeholders unacceptable Risk Control Risk Reduction Risk Acceptance Output / Result of the Quality Risk Management Process ICH Q 9 prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance Risk Management tools Risk Evaluation Risk Review Events July 2006, slide 16

Content ICH Q 9 QUALITY RISK MANAGEMENT 4. General Quality Risk Management Process Risk Assessment: Risk Analysis “What is the likelihood it will go wrong? ” · The estimation of the risk associated with the identified hazards. · A qualitative or quantitative process of linking the likelihood of occurrence and severity of harm Initiate Quality Risk Management Process Risk Assessment · Consider detectability if applicable (used in some tools) Risk Identification Risk Analysis prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance Risk Communication unacceptable Risk Control Risk Reduction Risk Acceptance Output / Result of the Quality Risk Management Process ICH Q 9 Risk Management tools Risk Evaluation Risk Review Events July 2006, slide 17

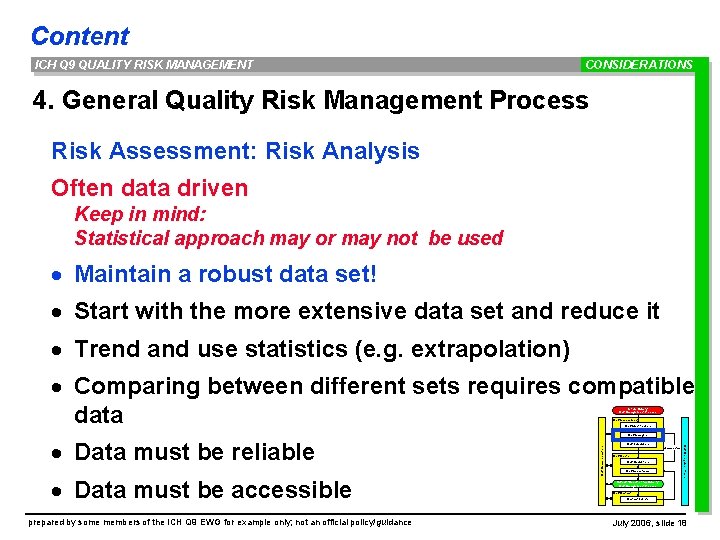
Content ICH Q 9 QUALITY RISK MANAGEMENT CONSIDERATIONS 4. General Quality Risk Management Process Risk Assessment: Risk Analysis Often data driven Keep in mind: Statistical approach may or may not be used · Maintain a robust data set! · Start with the more extensive data set and reduce it · Trend and use statistics (e. g. extrapolation) · Comparing between different sets requires compatible data Initiate Quality Risk Management Process Risk Assessment Risk Identification prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance Risk Communication · Data must be accessible Risk Management tools · Data must be reliable Risk Analysis Risk Evaluation unacceptable Risk Control Risk Reduction Risk Acceptance Output / Result of the Quality Risk Management Process Risk Review Events July 2006, slide 18

Content ICH Q 9 QUALITY RISK MANAGEMENT 4. General Quality Risk Management Process Risk Assessment: Risk Evaluation “What is the risk? ” · Compare the identified analysed risk against given risk criteria · Consider the strength of evidence for all three of the fundamental questions > What might go wrong? Initiate Quality Risk Management Process > What is the likelihood (probability) it will go wrong? Risk Assessment Risk Identification Risk Analysis Risk Communication unacceptable Risk Control Risk Reduction Risk Acceptance Output / Result of the Quality Risk Management Process Risk Review Events prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance Risk Management tools > What are the consequences (severity)? Risk Evaluation July 2006, slide 19

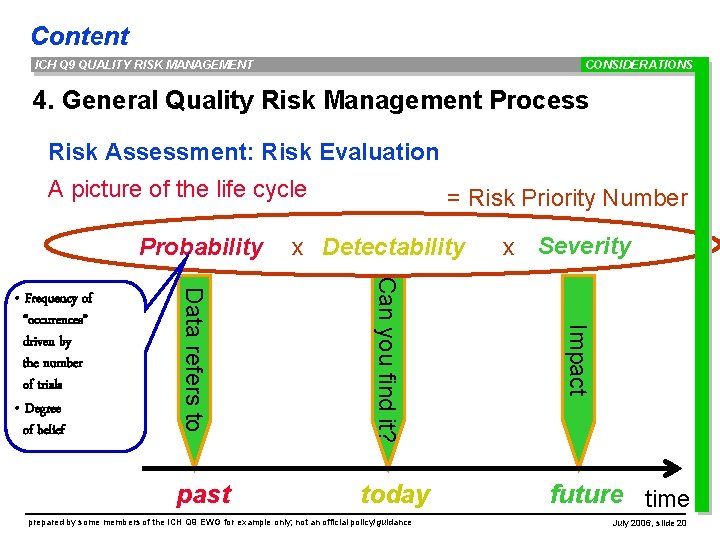
Content ICH Q 9 QUALITY RISK MANAGEMENT CONSIDERATIONS 4. General Quality Risk Management Process Risk Assessment: Risk Evaluation A picture of the life cycle Probability today prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance x Severity Impact past x Detectability Can you find it? Data refers to • Frequency of “occurences” driven by the number of trials • Degree of belief = Risk Priority Number future time July 2006, slide 20

Content ICH Q 9 QUALITY RISK MANAGEMENT 4. General Quality Risk Management Process Risk Control: Decision-making activity · Is the risk above an acceptable level? · What can be done to reduce or eliminate risks? · What is the appropriate balance between benefits, risks and resources? · Are new risks introduced as a result of the identified risks being controlled? Initiate Quality Risk Management Process Risk Assessment Risk Identification Risk Analysis Risk Communication unacceptable Risk Control Risk Reduction Risk Acceptance Output / Result of the Quality Risk Management Process ICH Q 9 prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance Risk Management tools Risk Evaluation Risk Review Events July 2006, slide 21

Content ICH Q 9 QUALITY RISK MANAGEMENT CONSIDERATIONS 4. General Quality Risk Management Process Risk Control: Residual Risk · The residual risk consists of e. g. > Hazards that have been assessed and risks that have been accepted > Hazards which have been identified but the risks have not been correctly assessed > Hazards that have not yet been identified > Hazards which are not yet linked to the patient risk · Is the risk reduced to an acceptable level? Risk Identification Risk Analysis Risk Communication Risk Evaluation unacceptable Risk Control Risk Reduction Risk Acceptance Output / Result of the Quality Risk Management Process Risk Review Events prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance Risk Management tools > Fulfil all legal and internal obligations > Consider current scientific knowledge & techniques Initiate Quality Risk Management Process Risk Assessment July 2006, slide 22

Content ICH Q 9 QUALITY RISK MANAGEMENT 4. General Quality Risk Management Process Risk Control: Risk Reduction · Mitigation or avoidance of quality risk · Elimination of risks, where appropriate · Focus actions on severity and/or probability of harm; don’t forget detectability · It might be appropriate to revisit the risk assessment during the life cycle for new risks or increased significance of existing risks Initiate Quality Risk Management Process Risk Assessment Risk Identification Risk Analysis Risk Communication unacceptable Risk Control Risk Reduction Risk Acceptance Output / Result of the Quality Risk Management Process ICH Q 9 prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance Risk Management tools Risk Evaluation Risk Review Events July 2006, slide 23

Content ICH Q 9 QUALITY RISK MANAGEMENT 4. General Quality Risk Management Process Risk Control: Risk Acceptance · Decision to > Accept the residual risk > Passively accept non specified residual risks · May require support by (senior) management > Applies to both industry and competent authorities Initiate Quality Risk Management Process Risk Assessment Risk Identification Risk Analysis Risk Communication unacceptable Risk Control Risk Reduction Risk Acceptance Output / Result of the Quality Risk Management Process Risk Review Events prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance Risk Management tools · Will always be made on a case-by-case basis Risk Evaluation July 2006, slide 24

Content ICH Q 9 QUALITY RISK MANAGEMENT CONSIDERATIONS 4. General Quality Risk Management Process Risk Control: Risk Acceptance · Discuss the appropriate balance between benefits, risks, and resources · Focus on the patients’ interests and good science/data · Risk acceptance is not Initiate Quality Risk Management Process Risk Assessment Risk Identification Risk Analysis Risk Communication Risk Evaluation Risk Management tools > Inappropriately interpreting data and information > Hiding risks from management / competent authorities unacceptable Risk Control Risk Reduction Risk Acceptance Output / Result of the Quality Risk Management Process Risk Review Events prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 25

Content ICH Q 9 QUALITY RISK MANAGEMENT What is an “acceptable risk”? Risk Control: Risk Acceptance Who has to accept risk? · Decision Maker(s) > Person(s) with the competence and authority to make appropriate and timely quality risk management decisions · Stakeholder > Any individual, group or organization that can …be affected by a risk > Decision makers might also be stakeholders > The primary stakeholders are the patient, healthcare professional, regulatory authority, and industry (ICH Q 9, definition) > The secondary stakeholders are patient associations, public opinions, politicians prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 26

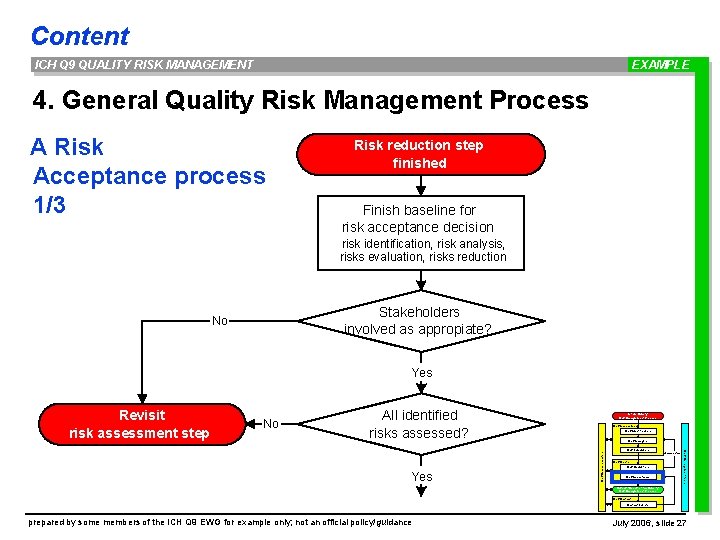
Content ICH Q 9 QUALITY RISK MANAGEMENT EXAMPLE 4. General Quality Risk Management Process A Risk Acceptance process 1/3 Risk reduction step finished Finish baseline for risk acceptance decision risk identification, risk analysis, risks evaluation, risks reduction Stakeholders involved as appropiate? No Yes Revisit risk assessment step No All identified risks assessed? Initiate Quality Risk Management Process Risk Assessment Risk Identification Risk Analysis Risk Communication Yes Risk Management tools Risk Evaluation unacceptable Risk Control Risk Reduction Risk Acceptance Output / Result of the Quality Risk Management Process Risk Review Events prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 27

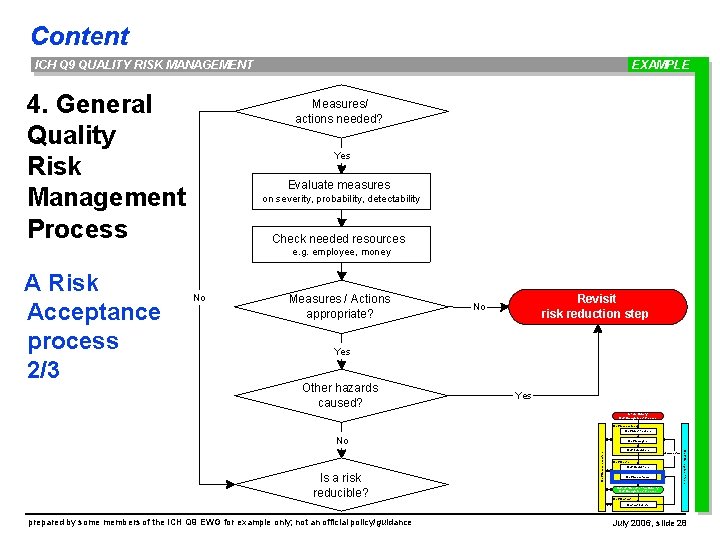
Content ICH Q 9 QUALITY RISK MANAGEMENT 4. General Quality Risk Management Process EXAMPLE Measures/ actions needed? Yes Evaluate measures on severity, probability, detectability Check needed resources e. g. employee, money A Risk Acceptance process 2/3 No Measures / Actions appropriate? Revisit risk reduction step No Yes Other hazards caused? Yes Initiate Quality Risk Management Process Risk Assessment Risk Identification No Risk Analysis Risk Communication Is a risk reducible? Risk Management tools Risk Evaluation unacceptable Risk Control Risk Reduction Risk Acceptance Output / Result of the Quality Risk Management Process Risk Review Events prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 28

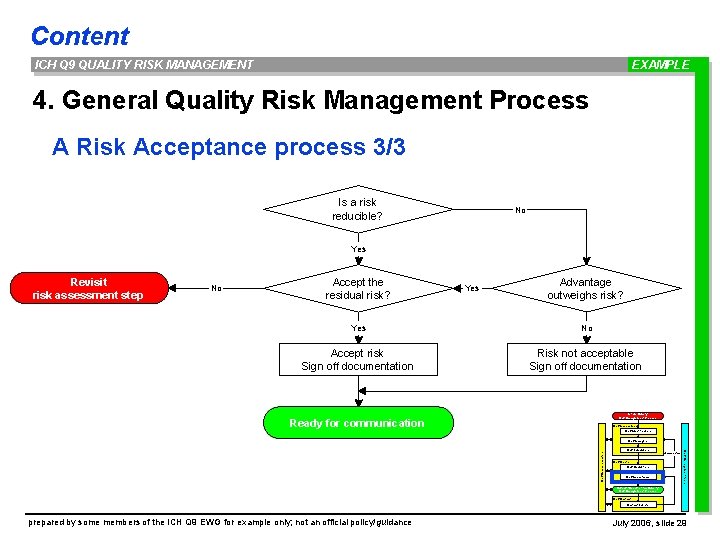
Content ICH Q 9 QUALITY RISK MANAGEMENT EXAMPLE 4. General Quality Risk Management Process A Risk Acceptance process 3/3 Is a risk reducible? No Yes Revisit risk assessment step No Accept the residual risk? Yes Advantage outweighs risk? Yes No Accept risk Sign off documentation Risk not acceptable Sign off documentation Initiate Quality Risk Management Process Ready for communication Risk Assessment Risk Identification Risk Analysis Risk Communication Risk Management tools Risk Evaluation unacceptable Risk Control Risk Reduction Risk Acceptance Output / Result of the Quality Risk Management Process Risk Review Events prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 29

Content ICH Q 9 QUALITY RISK MANAGEMENT 4. General Quality Risk Management Process Risk Communication · Bi-directional sharing of information about risk and risk management between the decision makers and others · Communicate at any stage of the QRM process · Communicate and document the output/result of the QRM process appropriately · Communication need not be carried out for each and every individual risk acceptance Initiate Quality Risk Management Process Risk Assessment Risk Identification Risk Analysis According to ICH Q 9 prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance Risk Communication Risk Management tools · Use existing channels as specified in regulations, guidance and SOP’s Risk Evaluation unacceptable Risk Control Risk Reduction Risk Acceptance Output / Result of the Quality Risk Management Process Risk Review Events July 2006, slide 30

Content ICH Q 9 QUALITY RISK MANAGEMENT CONSIDERATIONS 4. General Quality Risk Management Process Risk Communication · Exchange or sharing of information, as appropriate · Sometimes formal sometimes informal > Improve ways of thinking and communicating · Increase transparency Initiate Quality Risk Management Process Risk Assessment Risk Identification Risk Analysis Risk Communication Risk Management tools Risk Evaluation unacceptable Risk Control Risk Reduction Risk Acceptance Output / Result of the Quality Risk Management Process Risk Review Events prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 31

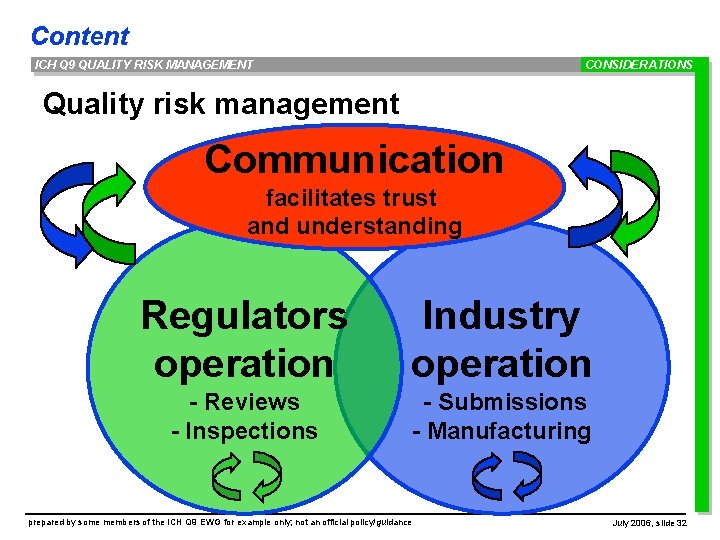
Content ICH Q 9 QUALITY RISK MANAGEMENT CONSIDERATIONS Quality risk management Communication facilitates trust and understanding Regulators operation Industry operation - Reviews - Inspections - Submissions - Manufacturing prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 32

Content ICH Q 9 QUALITY RISK MANAGEMENT 4. General Quality Risk Management Process Risk review: Review Events · Review the output / results of the QRM process · Take into account new knowledge and experience · Utilise for planned or unplanned events · Implement a mechanism to review or monitor events · Reconsideration of risk acceptance decisions, as appropriate Initiate Quality Risk Management Process Risk Assessment Risk Identification Risk Analysis Risk Communication unacceptable Risk Control Risk Reduction Risk Acceptance Output / Result of the Quality Risk Management Process ICH Q 9 prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance Risk Management tools Risk Evaluation Risk Review Events July 2006, slide 33

Content ICH Q 9 QUALITY RISK MANAGEMENT 5. Risk Management Methodology One method “all inclusive”? Initiate Quality Risk Management Process Risk Assessment Risk Identification Risk Analysis Risk Communication unacceptable Risk Control Risk Reduction Risk Acceptance Output / Result of the Quality Risk Management Process Risk Review Cartoon: © Zurich Insurance Inc. prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance Risk Management tools Risk Evaluation Review Events July 2006, slide 34

Content ICH Q 9 QUALITY RISK MANAGEMENT CONSIDERATIONS Expectations on methods and tools · Supports science-based decisions · A great variety are listed but other existing or new ones might also be used · No single tool is appropriate for all cases · Specific risks do not always require the same tool · Using a tool the level of detail of an investigation will vary according to the risk from case to case · Different companies, consultancies and competent authorities may promote use of different tools based on their culture and experiences prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 35

Content ICH Q 9 QUALITY RISK MANAGEMENT CONSIDERATIONS Contributing items to manage quality risks · System Risk (facility & people) > e. g. interfaces, operators risk, environment, components such as equipment, IT, design elements · System Risk (organisation) > e. g. Quality systems, controls, measurements, documentation, regulatory compliance · Process Risk > e. g. process operations and quality parameters · Product Risk (safety & efficacy) > e. g. quality attributes: measured data according to specifications prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 36

Content ICH Q 9 QUALITY RISK MANAGEMENT 5. Risk Management Methodology · Supports a scientific and practical approach to decision-making · Accomplishing steps of the QRM process > Provides documented, transparent and reproducible methods > Assessing current knowledge > Assessing probability, severity and sometimes detectability Initiate Quality Risk Management Process Risk Assessment Risk Identification Risk Analysis Risk Communication unacceptable Risk Control Risk Reduction Risk Acceptance Output / Result of the Quality Risk Management Process ICH Q 9 prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance Risk Management tools Risk Evaluation Risk Review Events July 2006, slide 37

Content ICH Q 9 QUALITY RISK MANAGEMENT 5. Risk Management Methodology · Adapt the tools for use in specific areas · Combined use of tools may provide flexibility · The degree of rigor and formality of QRM > Should be commensurate with the complexity and / or criticality of the issue to be addressed and reflect available knowledge · Informal ways Initiate Quality Risk Management Process Risk Assessment > empirical methods and / or internal procedures Risk Identification Risk Analysis Risk Communication unacceptable Risk Control Risk Reduction Risk Acceptance Output / Result of the Quality Risk Management Process ICH Q 9 prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance Risk Management tools Risk Evaluation Risk Review Events July 2006, slide 38

Content ICH Q 9 QUALITY RISK MANAGEMENT Annex I: Risk Management Methods and Tools · Provides a general overview of and references for some of the primary tools · Might be used in QRM by industry and regulators · This is not an exhaustive list · No one tool or set of tools is applicable to every situation in which a QRM procedure is used · For each of the tools > Short description & reference > Strength and weaknesses > Purely illustrative examples Initiate Quality Risk Management Process Risk Assessment Risk Identification Risk Analysis prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance Risk Communication unacceptable Risk Control Risk Reduction Risk Acceptance Output / Result of the Quality Risk Management Process ICH Q 9 Risk Management tools Risk Evaluation Risk Review Events July 2006, slide 39

Content ICH Q 9 QUALITY RISK MANAGEMENT CONSIDERATIONS Overview: Some tools and their key words · Failure Mode Effects Analysis (FMEA) > Break down large complex processes into manageable steps · Failure Mode, Effects and Criticality Analysis (FMECA) > FMEA & links severity, probability & detectability to criticality · Fault Tree Analysis (FTA) > Tree of failure modes combinations with logical operators · Hazard Analysis and Critical Control Points (HACCP) > Systematic, proactive, and preventive method on criticality · Hazard Operability Analysis (HAZOP) > Brainstorming technique · Preliminary Hazard Analysis (PHA) > Compare and prioritize risks with factors for each risk prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance Risk Analysis Risk Evaluation Risk Communication · Risk ranking and filtering Risk Identification Risk Management tools > Possibilities that the risk event happens Initiate Quality Risk Management Process Risk Assessment unacceptable Risk Control Risk Reduction Risk Acceptance Output / Result of the Quality Risk Management Process Risk Review Events July 2006, slide 40

Content ICH Q 9 QUALITY RISK MANAGEMENT 5. Risk Management Methodology · Supporting statistical tools > Acceptance Control Charts (see ISO 7966) > Control Charts (for example) Ø Control Charts with Arithmetic Average and Warning Limits (see ISO 7873) Ø Cumulative Sum Charts; “Cu. Sum” (see ISO 7871) Ø Shewhart Control Charts (see ISO 8258) Ø Weighted Moving Average > Design of Experiments (DOE) Ø Pareto Charts > Process Capability Analysis Initiate Quality Risk Management Process Risk Assessment Risk Identification Risk Analysis prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance Risk Communication > Use others that you are familiar with…. unacceptable Risk Control Risk Reduction Risk Acceptance Output / Result of the Quality Risk Management Process ICH Q 9 Risk Management tools > Histograms Risk Evaluation Risk Review Events July 2006, slide 41

Content ICH Q 9 QUALITY RISK MANAGEMENT CONSIDERATIONS 5. Risk Management Methodology Q 9 does not provide “drivers licences” prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 42

Content ICH Q 9 QUALITY RISK MANAGEMENT 6. Integration into Industry and Regulatory Operations · Foundation for “science-based” decisions · Does not obviate industry’s obligation to comply with regulatory requirements · May affect the extent and level of direct regulatory oversight · Degree of rigor and formality commensurate with the complexity and/or criticality of the issue · Implement QRM principles when updating existing guidelines ICH Q 9 prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 43

Content ICH Q 9 QUALITY RISK MANAGEMENT Annex II: Potential Applications for QRM This Annex is intended to identify potential uses of quality risk management principles and tools by industry and regulators. However, the selection of particular risk management tools is completely dependent upon specific facts and circumstances. These examples are provided for illustrative purposes and only suggest potential uses of quality risk management. This Annex is not intended to create any new expectations beyond the current regulatory requirements. ICH Q 9 Introduction to Annex II prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 44

Content ICH Q 9 QUALITY RISK MANAGEMENT Annex II: Potential Applications for QRM Quality risk management as part of · Integrated quality management > Documentation > Training and education > Quality defects > Auditing / Inspection Competent authorities Industry > Periodic review > Change management / change control > Continual improvement prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 45

Content ICH Q 9 QUALITY RISK MANAGEMENT Annex II: Potential opportunities for conducting quality risk management Quality risk management as part of · Regulatory operations > Inspection and assessment activities Competent authorities · Industry operations > > > Development Facilities, equipment and utilities Materials management Production Laboratory control and stability testing Packaging and labelling prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance Industry Competent authorities July 2006, slide 46

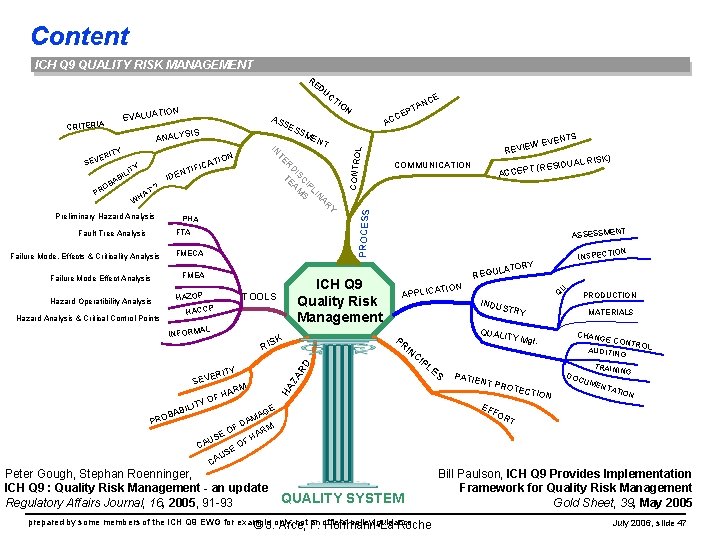
Content ICH Q 9 QUALITY RISK MANAGEMENT RE DU EV SIS CONTRO N LI IP C S IS M D EA T IDE `? AT AR WH Y Preliminary Hazard Analysis PHA FTA Fault Tree Analysis FMECA FMEA Hazard Operatibility Analysis HAZOP HACCP AL ) AL RISK (RESIDU ACCEPT T ASSESSMEN TION INSPEC TORY PRODUCTION MATERIALS QUALIT Y Mgt. CI CHANG E CON TROL AUDITING PL ES PATIE NT PR TRAININ G OTEC T DOC ION UME NTA TION EF FO E AG AM D M OF AR SE H U OF CA SE U CA Peter Gough, Stephan Roenninger, ICH Q 9 : Quality Risk Management - an update Regulatory Affairs Journal, 16, 2005, 91 -93 INDU S IN RD M U Q TRY ZA Y ERIT HAR ATION APPLIC PR K RIS F YO REGU ILIT BAB PRO ICH Q 9 Quality Risk Management TOOLS INFORM SEV TS EVEN LA Failure Mode Effect Analysis Hazard Analysis & Critical Control Points COMMUNICATION HA Failure Mode, Effects & Criticality Analysis W REVIE L NT R IL AB AC TE ON ATI C I F NTI ITY PT CE PROCESS TY ERI CE AN N ME ANALY B RO P AS SE SS IN SEV IO N ALUATIO CRITERIA CT RT QUALITY SYSTEM prepared by some members of the ICH Q 9 EWG for example not F. an official policy/guidance © J. only; Arce, Hoffmann-La Roche Bill Paulson, ICH Q 9 Provides Implementation Framework for Quality Risk Management Gold Sheet, 39, May 2005 July 2006, slide 47

Content Initiate Quality Risk Management Process ICH Q 9 QUALITY RISK MANAGEMENT Risk Assessment Risk Identification Risk Analysis Risk Evaluation Risk Communication Risk Control Risk Reduction Risk Acceptance Risk Management tools unacceptable Output / Result of the Quality Risk Management Process Risk Review Events Use the right “risk” expression please! prepared by some members of the ICH Q 9 EWG for example only; not an official policy/guidance July 2006, slide 48