Content 1 2 3 4 Atmospheric Composition Measures





- Slides: 24



Content 1 2 3 4 Atmospheric Composition Measures of Atmospheric Composition Atmospheric Structure Atmospheric Pressure

Definition Aerosol is a suspension of solid or liquid particles in a gas. • Atmospheric aerosols consist of small particles of liquid and solid material suspended in the air. • Bioaerosol: An aerosol of biological origin. (Examples: viruses, bacteria, fungi, spores, and pollens. )

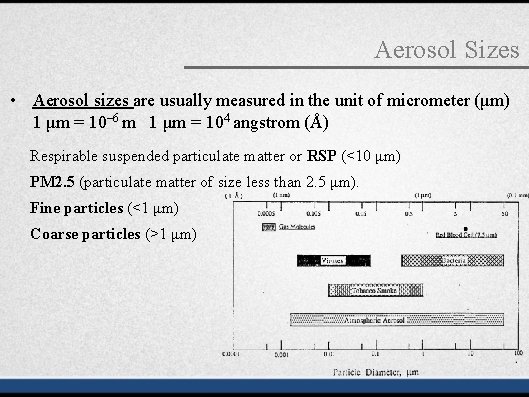
Aerosol Sizes • Aerosol sizes are usually measured in the unit of micrometer (μm) 1 μm = 10– 6 m 1 μm = 104 angstrom (Å) Respirable suspended particulate matter or RSP (<10 μm) PM 2. 5 (particulate matter of size less than 2. 5 μm). Fine particles (<1 μm) Coarse particles (>1 μm)

Air Quality and Aerosols Atmospheric Particles (Epidemiological evidence ) Diseases & Mortality – Particles penetrate the lungs, blocking and irritating air passages. – Particles themselves could exert toxic effects. (toxic substances present in the particles)

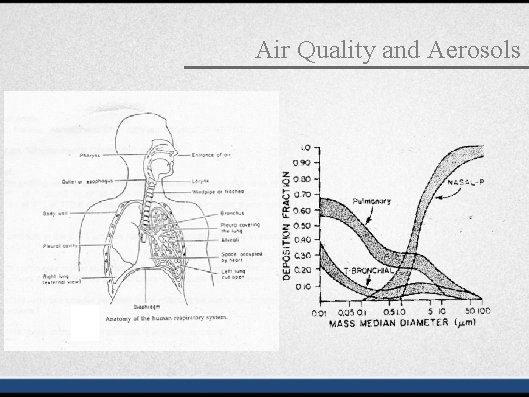
Air Quality and Aerosols

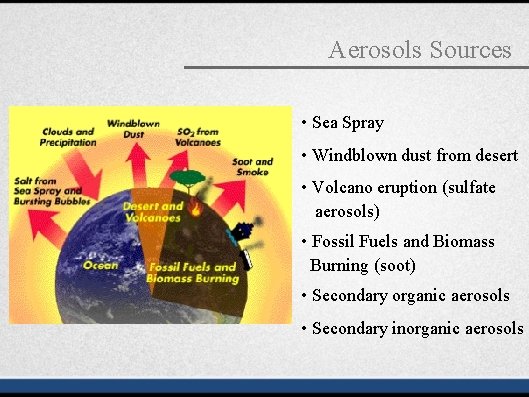
Aerosols Sources • Sea Spray • Windblown dust from desert • Volcano eruption (sulfate aerosols) • Fossil Fuels and Biomass Burning (soot) • Secondary organic aerosols • Secondary inorganic aerosols

Aerosols Residence Time in the Troposphere a) Pb-210 is produced by radioactive decay of 222 Rn, which is emitted from soils, and condenses immediately on preexisting aerosol particles. b) The 222 Rn emission flux is 1. 0 atoms cm-2 s-1 from land, which is 30% of the earth surface, and zero from the oceans. c) The only sink of 222 Rn is radioactive decay to produce 210 Pb (half life 3. 8 d) d) Removal of 210 Pb is by radioactive decay (half-life 23 years) and aerosol deposition. e) The total mass of 210 Pb in the troposphere is estimated to be 380 g. Question: What is the residence time against deposition of 210 Pb-carrying aerosols in the troposphere?

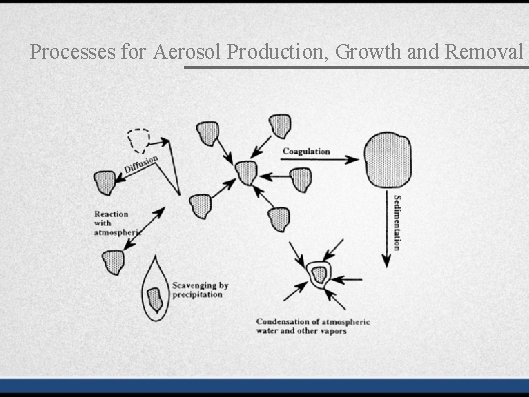
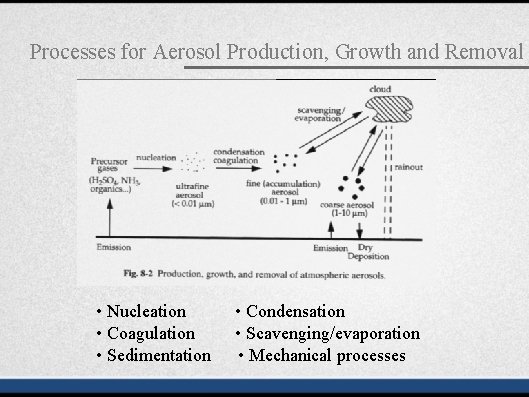
Processes for Aerosol Production, Growth and Removal

Processes for Aerosol Production, Growth and Removal • Nucleation • Coagulation • Sedimentation • Condensation • Scavenging/evaporation • Mechanical processes

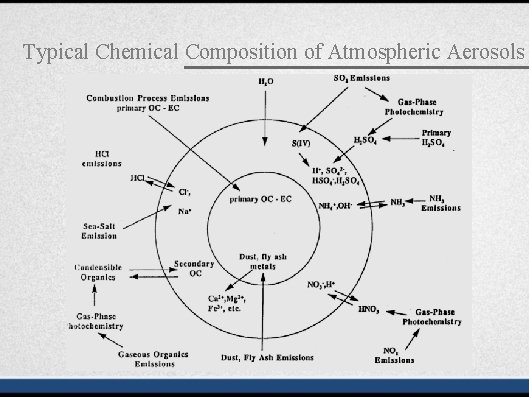
Typical Chemical Composition of Atmospheric Aerosols

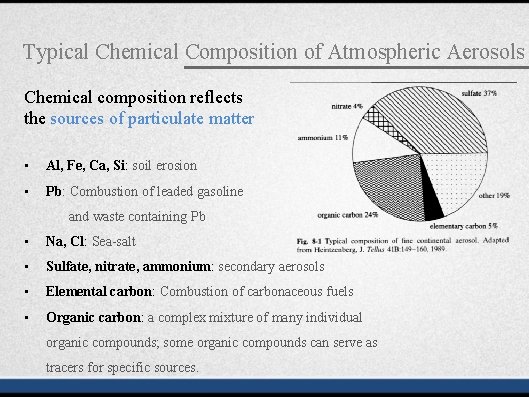
Typical Chemical Composition of Atmospheric Aerosols Chemical composition reflects the sources of particulate matter • Al, Fe, Ca, Si: soil erosion • Pb: Combustion of leaded gasoline and waste containing Pb • Na, Cl: Sea-salt • Sulfate, nitrate, ammonium: secondary aerosols • Elemental carbon: Combustion of carbonaceous fuels • Organic carbon: a complex mixture of many individual organic compounds; some organic compounds can serve as tracers for specific sources.

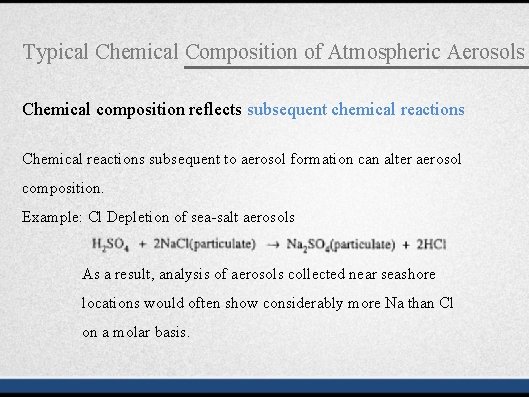
Typical Chemical Composition of Atmospheric Aerosols Chemical composition reflects subsequent chemical reactions Chemical reactions subsequent to aerosol formation can alter aerosol composition. Example: Cl Depletion of sea-salt aerosols As a result, analysis of aerosols collected near seashore locations would often show considerably more Na than Cl on a molar basis.

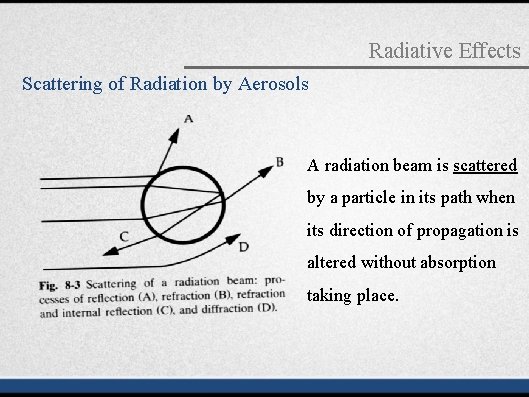
Radiative Effects Scattering of Radiation by Aerosols A radiation beam is scattered by a particle in its path when its direction of propagation is altered without absorption taking place.

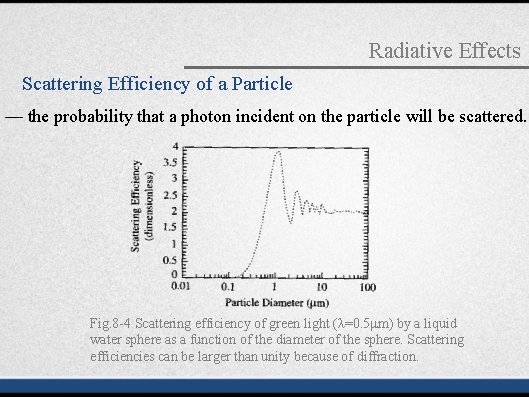
Radiative Effects Scattering Efficiency of a Particle — the probability that a photon incident on the particle will be scattered. Fig. 8 -4 Scattering efficiency of green light (λ=0. 5μm) by a liquid water sphere as a function of the diameter of the sphere. Scattering efficiencies can be larger than unity because of diffraction.

Radiative Effects Scattering Efficiency of a Particle • Scattering efficiency could be larger than 1 due to diffraction • Scattering efficiency is maximal for a particle radius corresponding to the wavelength of radiation. • Larger particles also scatter radiation efficiently, while smaller particles are inefficient scatters. • Particles in the accumulation mode (0. 1 -1μm) are efficient scatters of solar radiation.

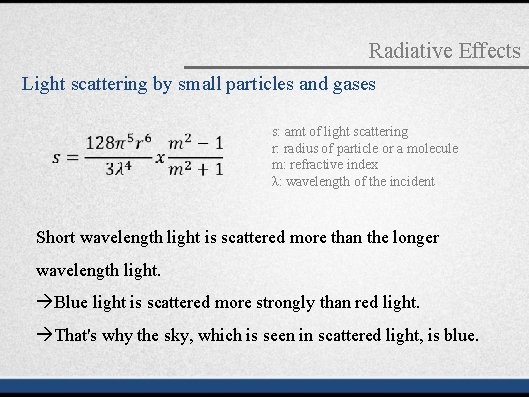
Radiative Effects Light scattering by small particles and gases s: amt of light scattering r: radius of particle or a molecule m: refractive index λ: wavelength of the incident Short wavelength light is scattered more than the longer wavelength light. Blue light is scattered more strongly than red light. That's why the sky, which is seen in scattered light, is blue.

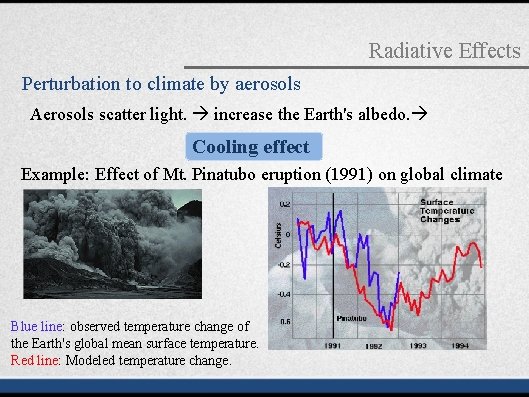
Radiative Effects Perturbation to climate by aerosols Aerosols scatter light. increase the Earth's albedo. Cooling effect Example: Effect of Mt. Pinatubo eruption (1991) on global climate Blue line: observed temperature change of the Earth's global mean surface temperature. Red line: Modeled temperature change.

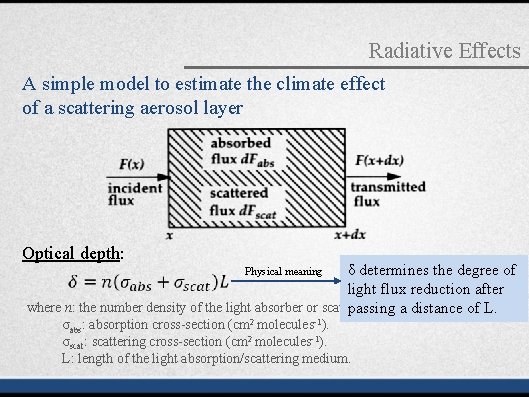
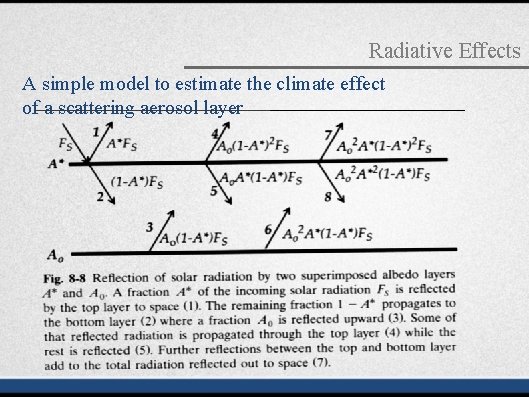
Radiative Effects A simple model to estimate the climate effect of a scattering aerosol layer Optical depth: δ determines the degree of light flux reduction after where n: the number density of the light absorber or scatterer passing a distance of L. Physical meaning σabs: absorption cross-section (cm 2 molecules-1). σscat: scattering cross-section (cm 2 molecules-1). L: length of the light absorption/scattering medium.

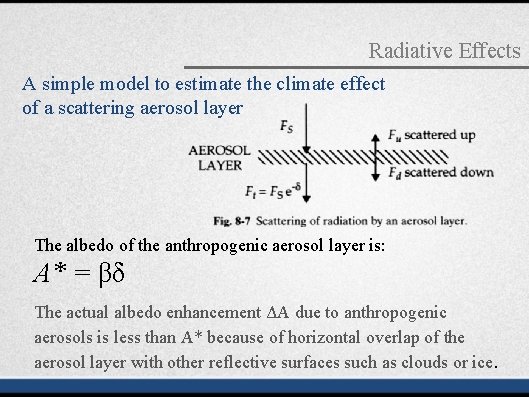
Radiative Effects A simple model to estimate the climate effect of a scattering aerosol layer The albedo of the anthropogenic aerosol layer is: A* = βδ The actual albedo enhancement ΔA due to anthropogenic aerosols is less than A* because of horizontal overlap of the aerosol layer with other reflective surfaces such as clouds or ice.

Radiative Effects A simple model to estimate the climate effect of a scattering aerosol layer

Radiative Effects Indirect Forcing 1. Aerosols serve as cloud condensation nuclei. o Cloud Condensation Nuclei (CCN) are particles that can become activated to grow to fog or cloud droplets in the presence of a supersaturation of water vapor. o If the Earth’s atmosphere were totally devoid of particles, clouds could not form.

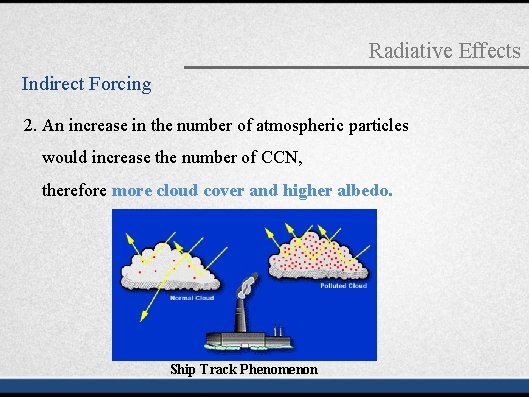
Radiative Effects Indirect Forcing 2. An increase in the number of atmospheric particles would increase the number of CCN, therefore more cloud cover and higher albedo. Ship Track Phenomenon