Contemporary Management of Patients With Advanced Hepatocellular Carcinoma




- Slides: 50

Contemporary Management of Patients With Advanced Hepatocellular Carcinoma Supported by educational grants from Astra. Zeneca, Genentech, a member of the Roche Group, and Lilly. For further information concerning Lilly grant funding visit www. lillygrantoffice. com.

About These Slides § Please feel free to use, update, and share some or all of these slides in your noncommercial presentations to colleagues or patients § When using our slides, please retain the source attribution: Slide credit: clinicaloptions. com § These slides may not be published, posted online, or used in commercial presentations without permission. Please contact permissions@clinicaloptions. com for details

Faculty Disclosures Richard Finn, MD Professor of Clinical Medicine Division of Hematology-Oncology Director, Signal Transduction and Therapeutics Program Jonsson Comprehensive Cancer Center David Geffen School of Medicine at UCLA Los Angeles, California Richard Finn, MD, has disclosed that he has received consulting fees from Astra. Zeneca, Bayer, Bristol-Myers Squibb, CStone, Eisai, Lilly, Merck, Pfizer, and Roche/Genentech.

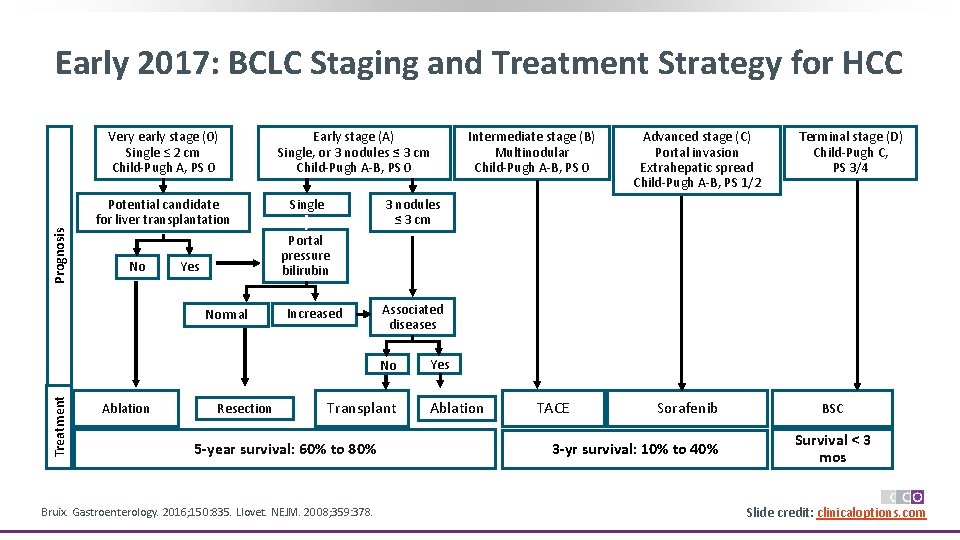
Early 2017: BCLC Staging and Treatment Strategy for HCC Prognosis Very early stage (0) Single ≤ 2 cm Child-Pugh A, PS 0 Potential candidate for liver transplantation No Early stage (A) Single, or 3 nodules ≤ 3 cm Child-Pugh A-B, PS 0 Single Terminal stage (D) Child-Pugh C, PS 3/4 3 nodules ≤ 3 cm Yes Increased Associated diseases No Treatment Advanced stage (C) Portal invasion Extrahepatic spread Child-Pugh A-B, PS 1/2 Portal pressure bilirubin Normal Ablation Intermediate stage (B) Multinodular Child-Pugh A-B, PS 0 Resection Transplant 5 -year survival: 60% to 80% Bruix. Gastroenterology. 2016; 150: 835. Llovet. NEJM. 2008; 359: 378. Yes Ablation TACE Sorafenib 3 -yr survival: 10% to 40% BSC Survival < 3 mos Slide credit: clinicaloptions. com

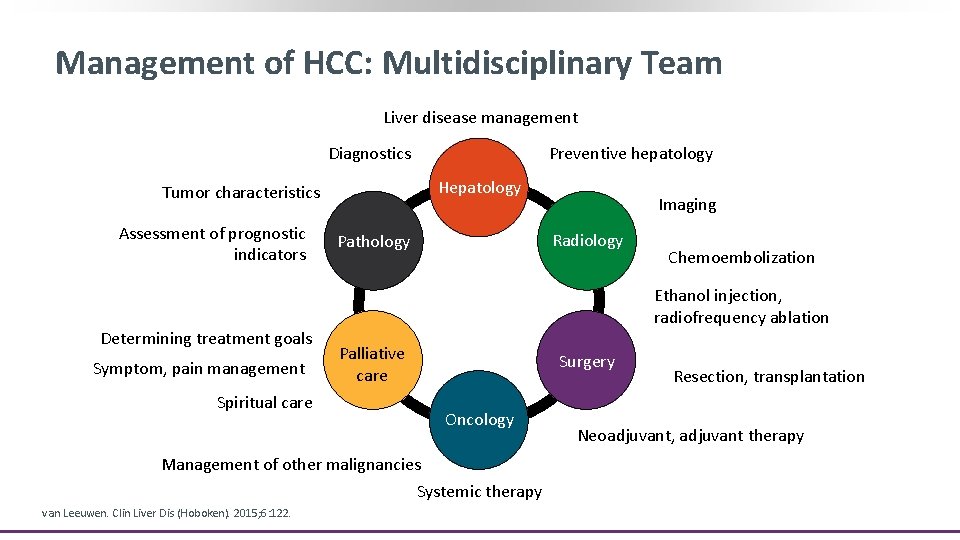
Management of HCC: Multidisciplinary Team Liver disease management Preventive hepatology Diagnostics Hepatology Tumor characteristics Assessment of prognostic indicators Determining treatment goals Symptom, pain management Radiology Pathology Liver tumor team Palliative care Oncology Management of other malignancies Systemic therapy Chemoembolization Ethanol injection, radiofrequency ablation Surgery Spiritual care van Leeuwen. Clin Liver Dis (Hoboken). 2015; 6: 122. Imaging Resection, transplantation Neoadjuvant, adjuvant therapy

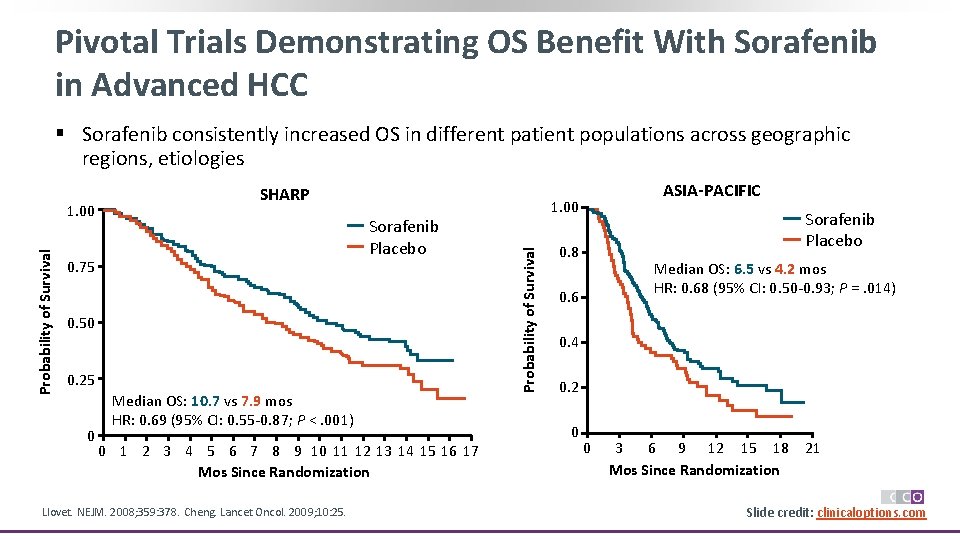
Pivotal Trials Demonstrating OS Benefit With Sorafenib in Advanced HCC § Sorafenib consistently increased OS in different patient populations across geographic regions, etiologies Sorafenib Placebo 0. 75 0. 50 0. 25 0 Median OS: 10. 7 vs 7. 9 mos HR: 0. 69 (95% CI: 0. 55 -0. 87; P <. 001) 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Mos Since Randomization Llovet. NEJM. 2008; 359: 378. Cheng. Lancet Oncol. 2009; 10: 25. ASIA-PACIFIC 1. 00 Probability of Survival 1. 00 SHARP Sorafenib Placebo 0. 8 Median OS: 6. 5 vs 4. 2 mos HR: 0. 68 (95% CI: 0. 50 -0. 93; P =. 014) 0. 6 0. 4 0. 2 0 0 3 6 9 12 15 18 Mos Since Randomization 21 Slide credit: clinicaloptions. com

REFLECT: Frontline Lenvatinib vs Sorafenib in Unresectable HCC § Randomized, open-label, noninferiority phase III trial ‒ Lenvatinib: multitargeted TKI against VEGFR-1, -2, -3; FGFR 1 -4; PDGFRα; KIT; RET Adult patients with unresectable HCC, no prior systemic therapy, Child-Pugh A, BCLC stage B or C, ECOG PS 0/1† (N = 954) § Primary endpoint: OS Lenvatinib QD* (n = 478) Sorafenib 400 mg BID (n = 476) *Body weight < 60 kg, 8 mg; body weight ≥ 60 kg, 12 mg. § Secondary endpoints: PFS, TTP, ORR, PK, Qo. L †Exclusion criteria included 50% or higher liver occupation, obvious invasion of bile duct, or invasion at main portal vein. Kudo. Lancet. 2018; 391: 1163. Lenvatinib PI. Slide credit: clinicaloptions. com

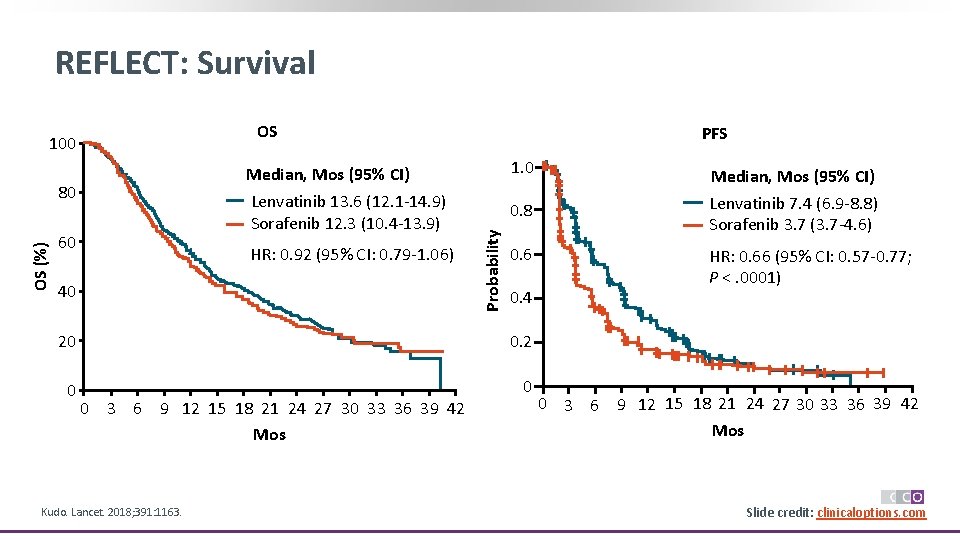
REFLECT: Survival OS Median, Mos (95% CI) Lenvatinib 13. 6 (12. 1 -14. 9) Sorafenib 12. 3 (10. 4 -13. 9) OS (%) 80 60 HR: 0. 92 (95% CI: 0. 79 -1. 06) 40 PFS 1. 0 0. 6 0. 2 0 0 Kudo. Lancet. 2018; 391: 1163. HR: 0. 66 (95% CI: 0. 57 -0. 77; P <. 0001) 0. 4 20 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 Mos Median, Mos (95% CI) Lenvatinib 7. 4 (6. 9 -8. 8) Sorafenib 3. 7 (3. 7 -4. 6) 0. 8 Probability 100 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 Mos *P <. 00001 vs sorafenib. Slide credit: clinicaloptions. com

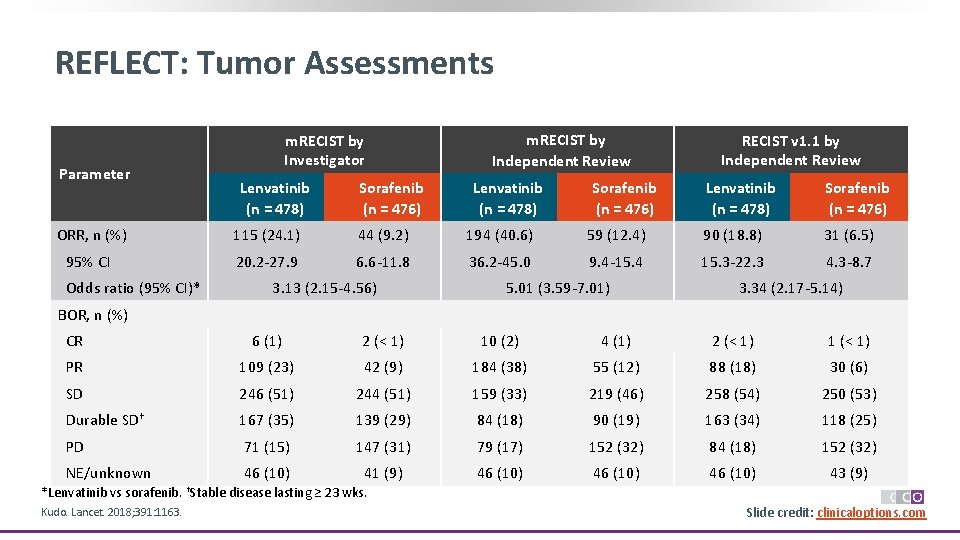
REFLECT: Tumor Assessments Parameter m. RECIST by Investigator Lenvatinib (n = 478) Sorafenib (n = 476) m. RECIST by Independent Review Lenvatinib (n = 478) Sorafenib (n = 476) RECIST v 1. 1 by Independent Review Lenvatinib (n = 478) Sorafenib (n = 476) ORR, n (%) 115 (24. 1) 44 (9. 2) 194 (40. 6) 59 (12. 4) 90 (18. 8) 31 (6. 5) 95% CI 20. 2 -27. 9 6. 6 -11. 8 36. 2 -45. 0 9. 4 -15. 4 15. 3 -22. 3 4. 3 -8. 7 Odds ratio (95% CI)* 3. 13 (2. 15 -4. 56) 5. 01 (3. 59 -7. 01) 3. 34 (2. 17 -5. 14) BOR, n (%) CR 6 (1) 2 (< 1) 10 (2) 4 (1) 2 (< 1) 1 (< 1) PR 109 (23) 42 (9) 184 (38) 55 (12) 88 (18) 30 (6) SD 246 (51) 244 (51) 159 (33) 219 (46) 258 (54) 250 (53) Durable SD† 167 (35) 139 (29) 84 (18) 90 (19) 163 (34) 118 (25) PD 71 (15) 147 (31) 79 (17) 152 (32) 84 (18) 152 (32) NE/unknown 46 (10) 41 (9) 46 (10) 43 (9) *Lenvatinib vs sorafenib. †Stable disease lasting ≥ 23 wks. Kudo. Lancet. 2018; 391: 1163. Slide credit: clinicaloptions. com

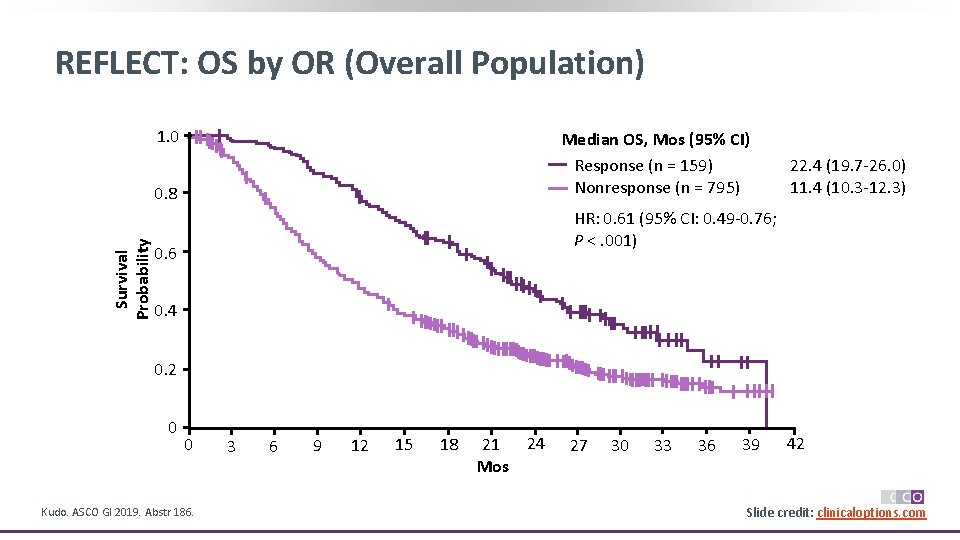
REFLECT: OS by OR (Overall Population) 1. 0 Median OS, Mos (95% CI) Response (n = 159) Nonresponse (n = 795) Survival Probability 0. 8 22. 4 (19. 7 -26. 0) 11. 4 (10. 3 -12. 3) HR: 0. 61 (95% CI: 0. 49 -0. 76; P <. 001) 0. 6 0. 4 0. 2 0 0 Kudo. ASCO GI 2019. Abstr 186. 3 6 9 12 15 18 21 24 Mos 27 30 33 36 39 42 Slide credit: clinicaloptions. com

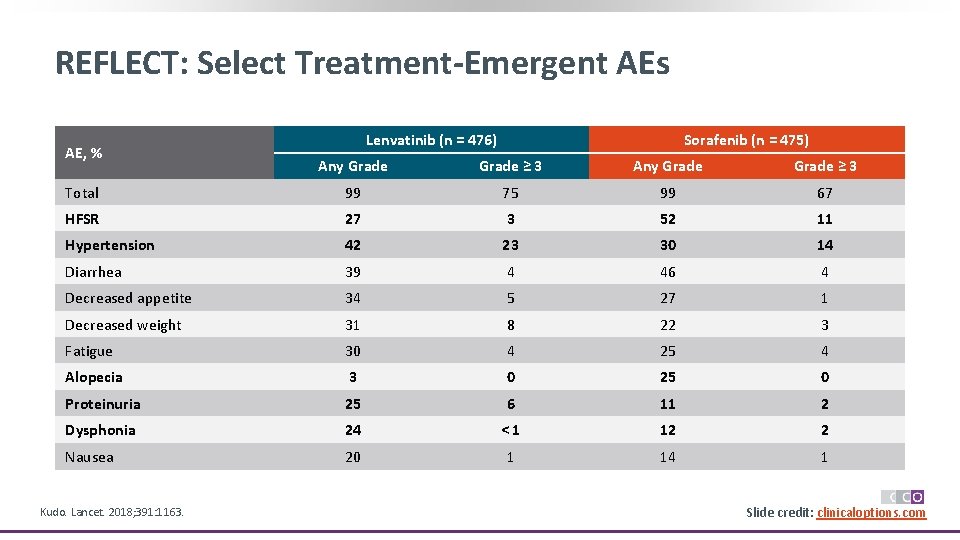
REFLECT: Select Treatment-Emergent AEs AE, % Question Lenvatinib (n = 476) Sorafenib (n = 475) Any Grade ≥ 3 Total 99 75 99 67 HFSR 27 3 52 11 Hypertension 42 23 30 14 Diarrhea 39 4 46 4 Decreased appetite 34 5 27 1 Decreased weight 31 8 22 3 Fatigue 30 4 25 4 Alopecia 3 0 25 0 Proteinuria 25 6 11 2 Dysphonia 24 <1 12 2 Nausea 20 1 14 1 Kudo. Lancet. 2018; 391: 1163. Slide credit: clinicaloptions. com

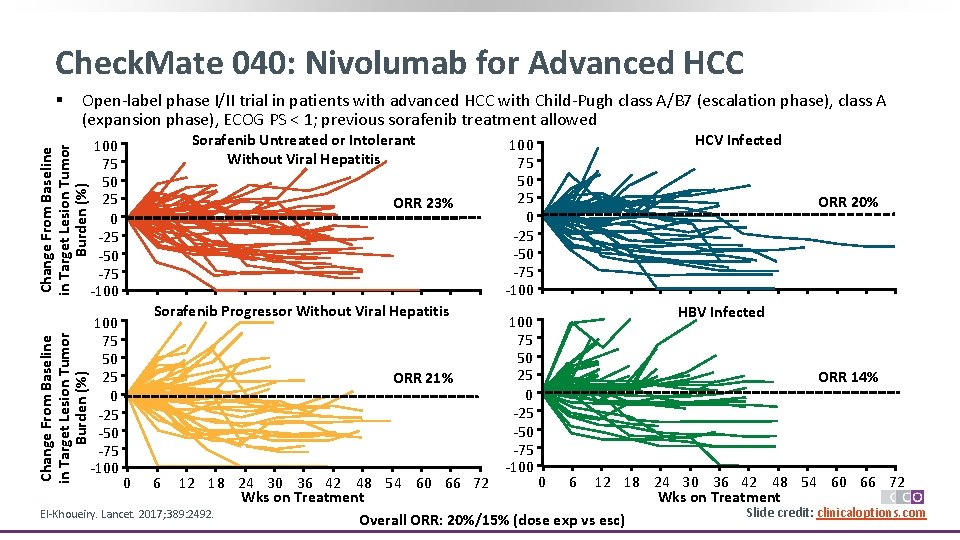
Check. Mate 040: Nivolumab for Advanced HCC § Open-label phase I/II trial in patients with advanced HCC with Child-Pugh class A/B 7 (escalation phase), class A (expansion phase), ECOG PS < 1; previous sorafenib treatment allowed Sorafenib Untreated or Intolerant Without Viral Hepatitis Change From Baseline in Target Lesion Tumor Burden (%) 100 75 50 25 0 -25 -50 -75 -100 ORR 23% Sorafenib Progressor Without Viral Hepatitis ORR 21% 100 75 50 25 0 -25 -50 -75 -100 HCV Infected ORR 20% HBV Infected ORR 14% 0 6 12 18 24 30 36 42 48 54 60 66 72 Wks on Treatment Slide credit: clinicaloptions. com El-Khoueiry. Lancet. 2017; 389: 2492. Overall ORR: 20%/15% (dose exp vs esc) 0 6

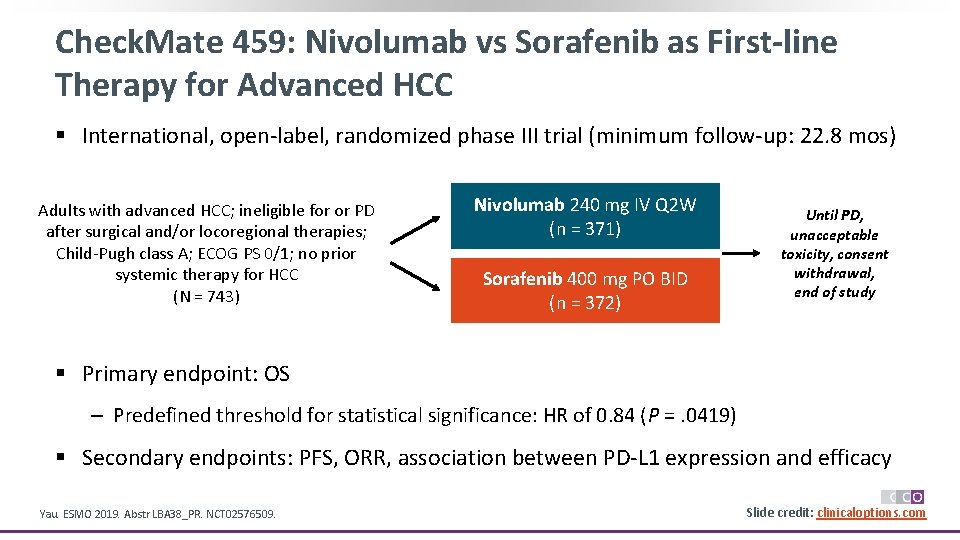
Check. Mate 459: Nivolumab vs Sorafenib as First-line Therapy for Advanced HCC § International, open-label, randomized phase III trial (minimum follow-up: 22. 8 mos) Adults with advanced HCC; ineligible for or PD after surgical and/or locoregional therapies; Child-Pugh class A; ECOG PS 0/1; no prior systemic therapy for HCC (N = 743) Nivolumab 240 mg IV Q 2 W (n = 371) Sorafenib 400 mg PO BID (n = 372) Until PD, unacceptable toxicity, consent withdrawal, end of study § Primary endpoint: OS ‒ Predefined threshold for statistical significance: HR of 0. 84 (P =. 0419) § Secondary endpoints: PFS, ORR, association between PD-L 1 expression and efficacy Yau. ESMO 2019. Abstr LBA 38_PR. NCT 02576509. Slide credit: clinicaloptions. com

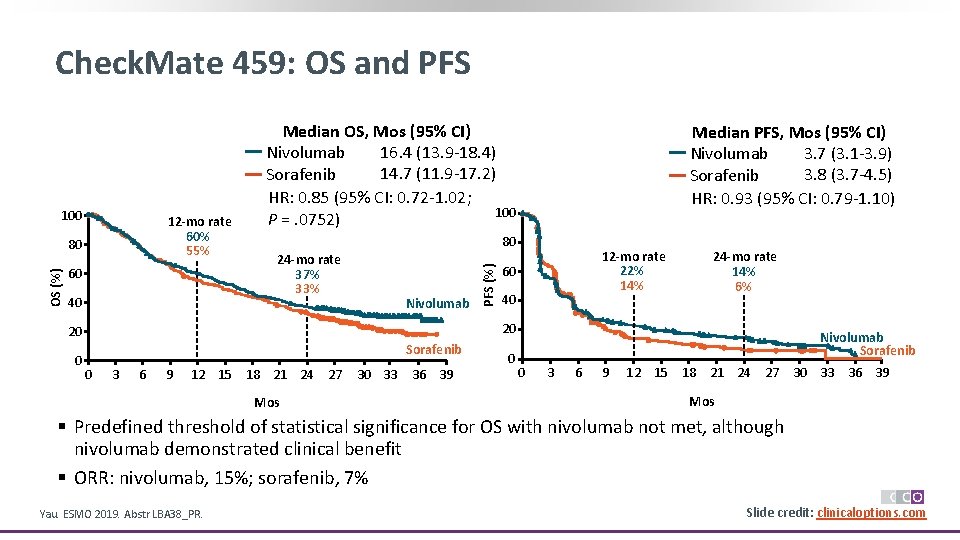
Check. Mate 459: OS and PFS 12 -mo rate 60% 55% OS (%) 80 60 40 24 -mo rate 37% 33% Nivolumab 12 -mo rate 22% 14% 60 40 24 -mo rate 14% 6% 20 20 0 Median PFS, Mos (95% CI) 3. 7 (3. 1 -3. 9) Nivolumab 3. 8 (3. 7 -4. 5) Sorafenib HR: 0. 93 (95% CI: 0. 79 -1. 10) 80 PFS (%) 100 Median OS, Mos (95% CI) 16. 4 (13. 9 -18. 4) Nivolumab 14. 7 (11. 9 -17. 2) Sorafenib HR: 0. 85 (95% CI: 0. 72 -1. 02; 100 P =. 0752) Sorafenib 0 3 6 9 12 15 18 21 24 27 Mos 30 33 36 39 0 Nivolumab Sorafenib 0 3 6 9 12 15 18 21 24 27 30 33 36 39 Mos § Predefined threshold of statistical significance for OS with nivolumab not met, although nivolumab demonstrated clinical benefit § ORR: nivolumab, 15%; sorafenib, 7% Yau. ESMO 2019. Abstr LBA 38_PR. Slide credit: clinicaloptions. com

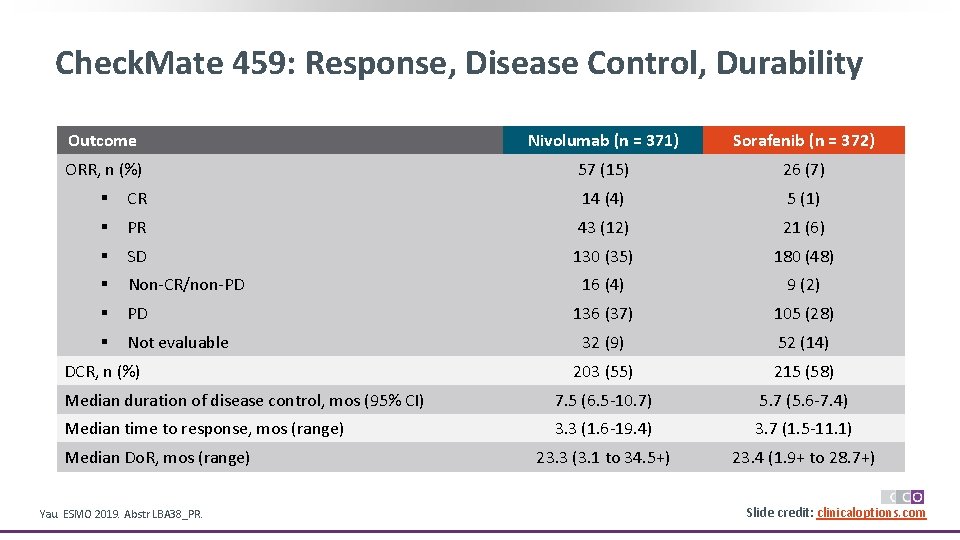
Check. Mate 459: Response, Disease Control, Durability Outcome Nivolumab (n = 371) Sorafenib (n = 372) ORR, n (%) 57 (15) 26 (7) § CR 14 (4) 5 (1) § PR 43 (12) 21 (6) § SD 130 (35) 180 (48) § Non-CR/non-PD 16 (4) 9 (2) § PD 136 (37) 105 (28) § Not evaluable 32 (9) 52 (14) 203 (55) 215 (58) Median duration of disease control, mos (95% CI) 7. 5 (6. 5 -10. 7) 5. 7 (5. 6 -7. 4) Median time to response, mos (range) 3. 3 (1. 6 -19. 4) 3. 7 (1. 5 -11. 1) 23. 3 (3. 1 to 34. 5+) 23. 4 (1. 9+ to 28. 7+) DCR, n (%) Median Do. R, mos (range) Yau. ESMO 2019. Abstr LBA 38_PR. Slide credit: clinicaloptions. com

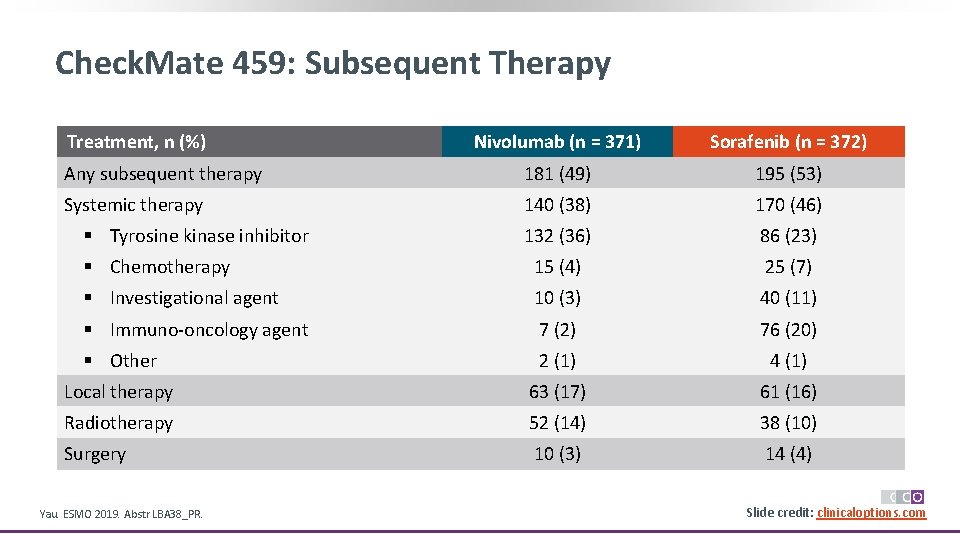
Check. Mate 459: Subsequent Therapy Treatment, n (%) Nivolumab (n = 371) Sorafenib (n = 372) Any subsequent therapy 181 (49) 195 (53) Systemic therapy 140 (38) 170 (46) 132 (36) 86 (23) § Chemotherapy 15 (4) 25 (7) § Investigational agent 10 (3) 40 (11) § Immuno-oncology agent 7 (2) 76 (20) § Other 2 (1) 4 (1) Local therapy 63 (17) 61 (16) Radiotherapy 52 (14) 38 (10) Surgery 10 (3) 14 (4) § Tyrosine kinase inhibitor Yau. ESMO 2019. Abstr LBA 38_PR. Slide credit: clinicaloptions. com

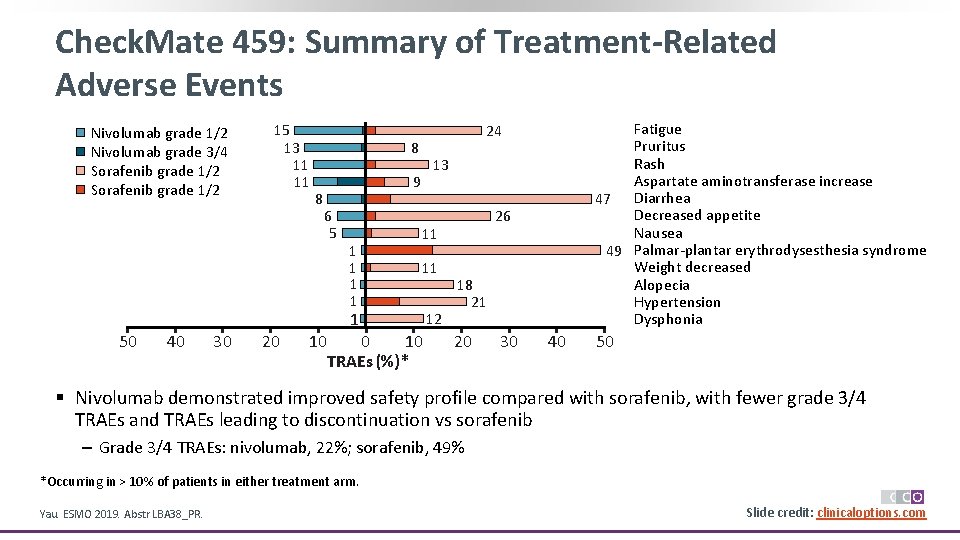
Check. Mate 459: Summary of Treatment-Related Adverse Events Nivolumab grade 1/2 Nivolumab grade 3/4 Sorafenib grade 1/2 15 13 11 11 8 8 13 9 6 5 1 1 40 30 20 10 26 11 11 1 50 Fatigue Pruritus Rash Aspartate aminotransferase increase 47 Diarrhea Decreased appetite Nausea 49 Palmar-plantar erythrodysesthesia syndrome Weight decreased Alopecia Hypertension Dysphonia 24 0 10 TRAEs (%)* 12 18 21 20 30 40 50 § Nivolumab demonstrated improved safety profile compared with sorafenib, with fewer grade 3/4 TRAEs and TRAEs leading to discontinuation vs sorafenib ‒ Grade 3/4 TRAEs: nivolumab, 22%; sorafenib, 49% *Occurring in > 10% of patients in either treatment arm. Yau. ESMO 2019. Abstr LBA 38_PR. Slide credit: clinicaloptions. com

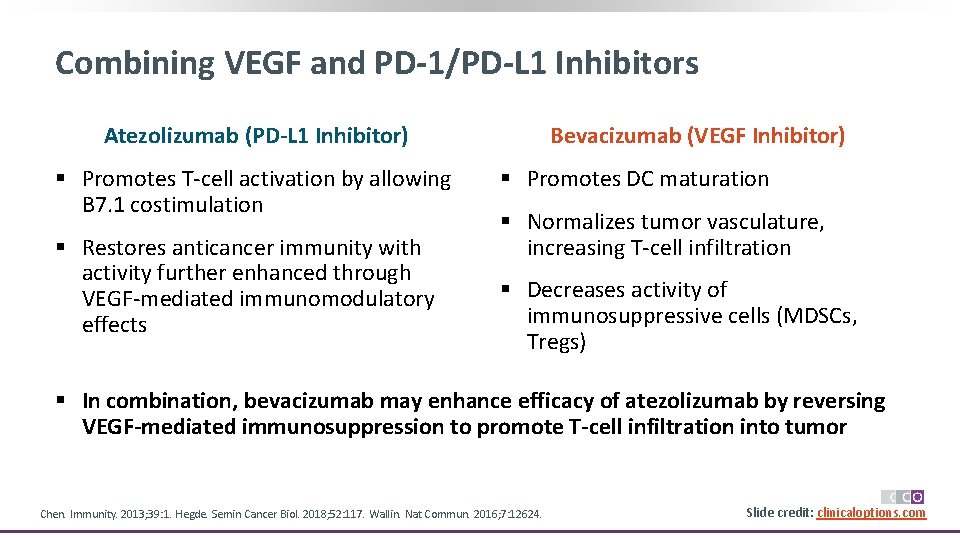
Combining VEGF and PD-1/PD-L 1 Inhibitors Atezolizumab (PD-L 1 Inhibitor) § Promotes T-cell activation by allowing B 7. 1 costimulation § Restores anticancer immunity with activity further enhanced through VEGF-mediated immunomodulatory effects Bevacizumab (VEGF Inhibitor) § Promotes DC maturation § Normalizes tumor vasculature, increasing T-cell infiltration § Decreases activity of immunosuppressive cells (MDSCs, Tregs) § In combination, bevacizumab may enhance efficacy of atezolizumab by reversing VEGF-mediated immunosuppression to promote T-cell infiltration into tumor Chen. Immunity. 2013; 39: 1. Hegde. Semin Cancer Biol. 2018; 52: 117. Wallin. Nat Commun. 2016; 7: 12624. Slide credit: clinicaloptions. com

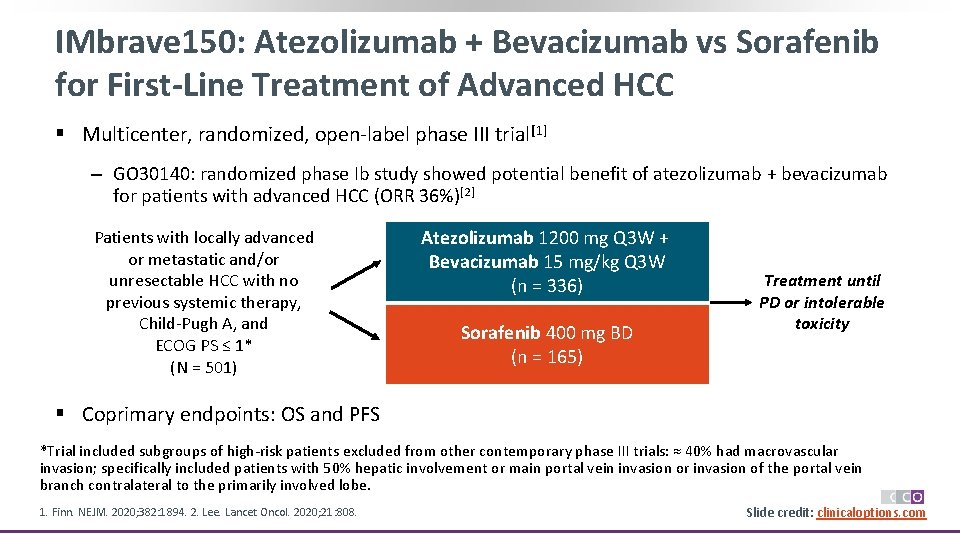
IMbrave 150: Atezolizumab + Bevacizumab vs Sorafenib for First-Line Treatment of Advanced HCC § Multicenter, randomized, open-label phase III trial[1] ‒ GO 30140: randomized phase Ib study showed potential benefit of atezolizumab + bevacizumab for patients with advanced HCC (ORR 36%)[2] Patients with locally advanced or metastatic and/or unresectable HCC with no previous systemic therapy, Child-Pugh A, and ECOG PS ≤ 1* (N = 501) Atezolizumab 1200 mg Q 3 W + Bevacizumab 15 mg/kg Q 3 W (n = 336) Sorafenib 400 mg BD (n = 165) Treatment until PD or intolerable toxicity § Coprimary endpoints: OS and PFS *Trial included subgroups of high-risk patients excluded from other contemporary phase III trials: ≈ 40% had macrovascular invasion; specifically included patients with 50% hepatic involvement or main portal vein invasion or invasion of the portal vein branch contralateral to the primarily involved lobe. 1. Finn. NEJM. 2020; 382: 1894. 2. Lee. Lancet Oncol. 2020; 21: 808. Slide credit: clinicaloptions. com

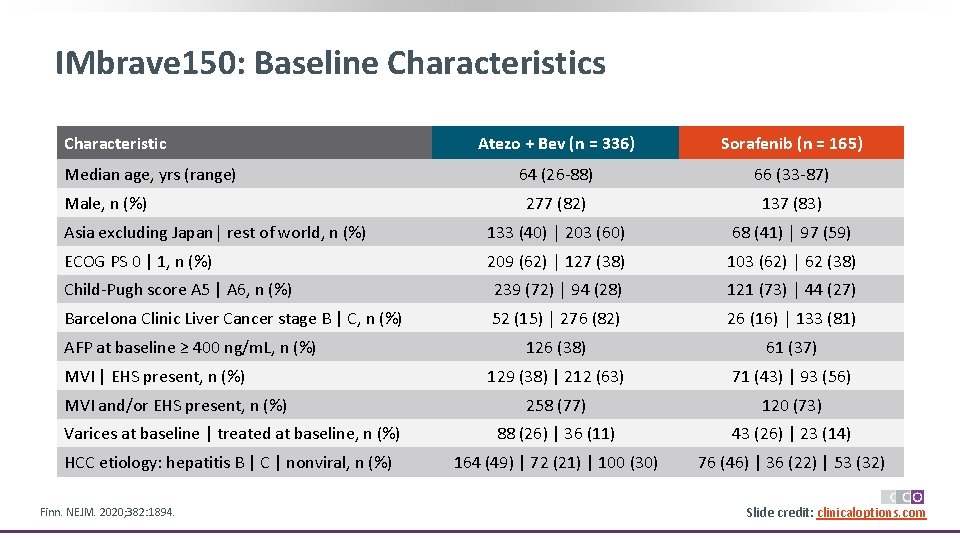
IMbrave 150: Baseline Characteristics Characteristic Atezo + Bev (n = 336) Sorafenib (n = 165) 64 (26 -88) 66 (33 -87) 277 (82) 137 (83) Asia excluding Japan│ rest of world, n (%) 133 (40) │ 203 (60) 68 (41) │ 97 (59) ECOG PS 0 | 1, n (%) 209 (62) │ 127 (38) 103 (62) │ 62 (38) Child-Pugh score A 5 | A 6, n (%) 239 (72) │ 94 (28) 121 (73) │ 44 (27) Barcelona Clinic Liver Cancer stage B | C, n (%) 52 (15) │ 276 (82) 26 (16) │ 133 (81) 126 (38) 61 (37) 129 (38) | 212 (63) 71 (43) | 93 (56) 258 (77) 120 (73) Varices at baseline | treated at baseline, n (%) 88 (26) | 36 (11) 43 (26) | 23 (14) HCC etiology: hepatitis B | C | nonviral, n (%) 164 (49) | 72 (21) | 100 (30) 76 (46) | 36 (22) | 53 (32) Median age, yrs (range) Male, n (%) AFP at baseline ≥ 400 ng/m. L, n (%) MVI | EHS present, n (%) MVI and/or EHS present, n (%) Finn. NEJM. 2020; 382: 1894. Slide credit: clinicaloptions. com

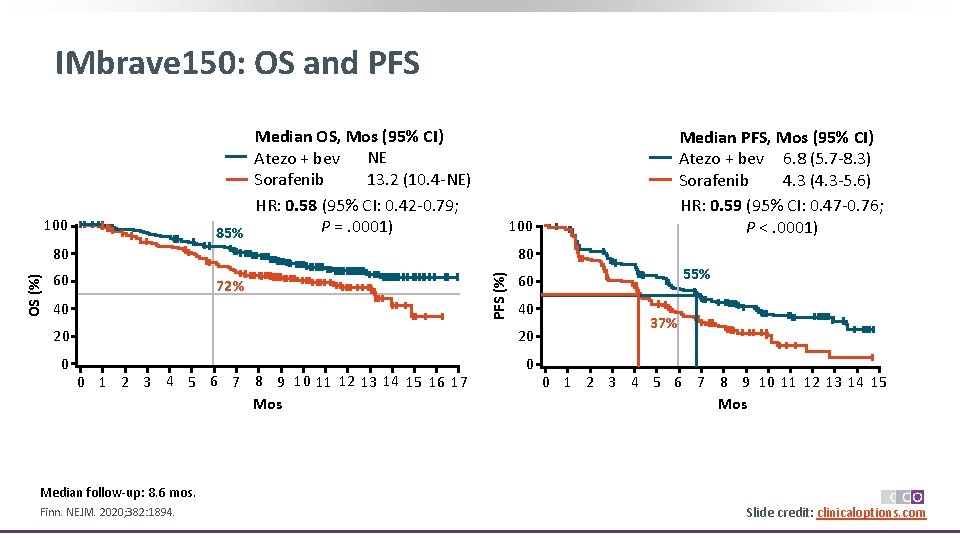
IMbrave 150: OS and PFS Median OS, Mos (95% CI) Atezo + bev NE 13. 2 (10. 4 -NE) Sorafenib HR: 0. 58 (95% CI: 0. 42 -0. 79; P =. 0001) 85% 100 80 80 60 72% 40 PFS (%) OS (%) Median PFS, Mos (95% CI) Atezo + bev 6. 8 (5. 7 -8. 3) 4. 3 (4. 3 -5. 6) Sorafenib HR: 0. 59 (95% CI: 0. 47 -0. 76; P <. 0001) 40 20 20 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Mos 55% 60 37% 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Mos Median follow-up: 8. 6 mos. Finn. NEJM. 2020; 382: 1894. Slide credit: clinicaloptions. com

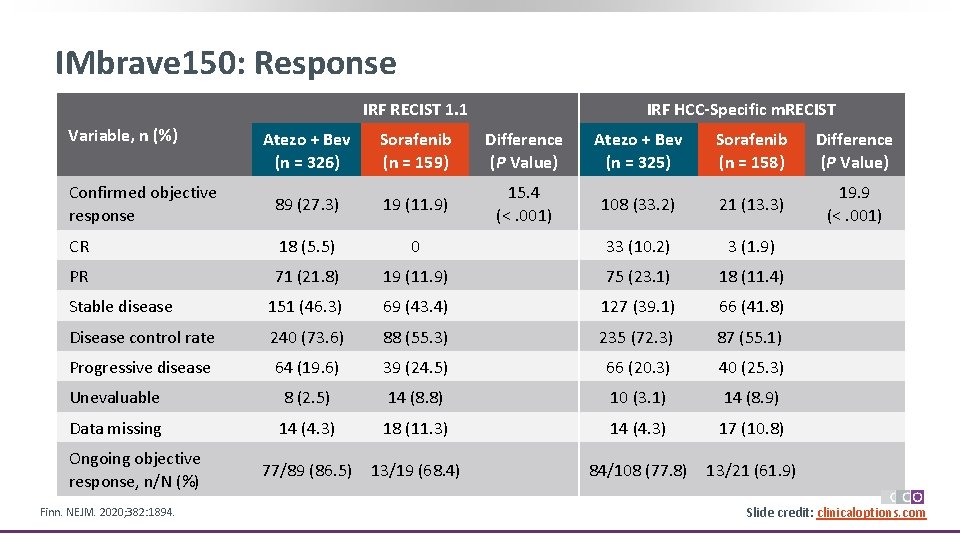
IMbrave 150: Response IRF RECIST 1. 1 Variable, n (%) IRF HCC-Specific m. RECIST Atezo + Bev (n = 326) Sorafenib (n = 159) Difference (P Value) Atezo + Bev (n = 325) Sorafenib (n = 158) Difference (P Value) Confirmed objective response 89 (27. 3) 19 (11. 9) 15. 4 (<. 001) 108 (33. 2) 21 (13. 3) 19. 9 (<. 001) CR 18 (5. 5) 0 33 (10. 2) 3 (1. 9) PR 71 (21. 8) 19 (11. 9) 75 (23. 1) 18 (11. 4) Stable disease 151 (46. 3) 69 (43. 4) 127 (39. 1) 66 (41. 8) Disease control rate 240 (73. 6) 88 (55. 3) 235 (72. 3) 87 (55. 1) Progressive disease 64 (19. 6) 39 (24. 5) 66 (20. 3) 40 (25. 3) Unevaluable 8 (2. 5) 14 (8. 8) 10 (3. 1) 14 (8. 9) Data missing 14 (4. 3) 18 (11. 3) 14 (4. 3) 17 (10. 8) 77/89 (86. 5) 13/19 (68. 4) 84/108 (77. 8) 13/21 (61. 9) Ongoing objective response, n/N (%) Finn. NEJM. 2020; 382: 1894. Slide credit: clinicaloptions. com

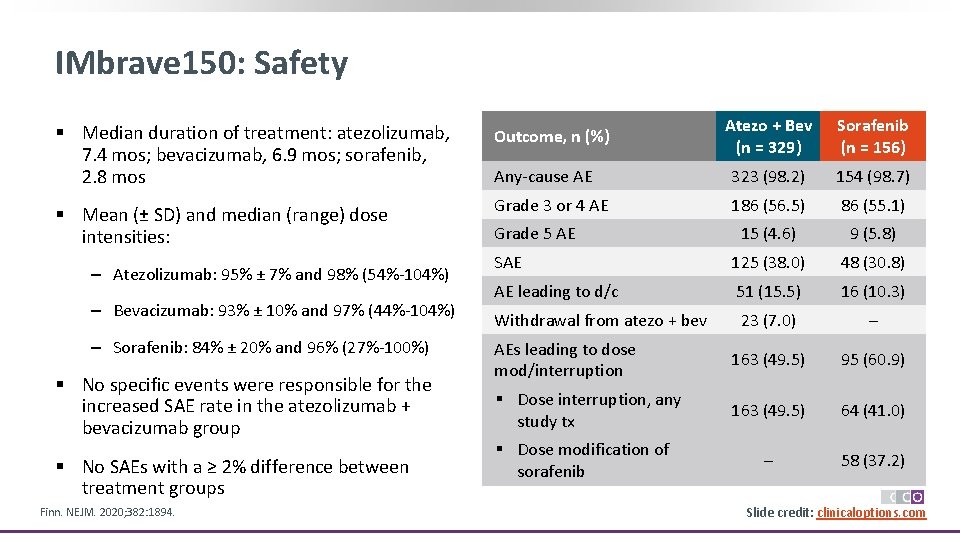
IMbrave 150: Safety Atezo + Bev (n = 329) Sorafenib (n = 156) Any-cause AE 323 (98. 2) 154 (98. 7) Grade 3 or 4 AE 186 (56. 5) 86 (55. 1) 15 (4. 6) 9 (5. 8) SAE 125 (38. 0) 48 (30. 8) AE leading to d/c 51 (15. 5) 16 (10. 3) Withdrawal from atezo + bev 23 (7. 0) – AEs leading to dose mod/interruption 163 (49. 5) 95 (60. 9) § Dose interruption, any study tx 163 (49. 5) 64 (41. 0) – 58 (37. 2) § Median duration of treatment: atezolizumab, 7. 4 mos; bevacizumab, 6. 9 mos; sorafenib, 2. 8 mos Outcome, n (%) § Mean (± SD) and median (range) dose intensities: ‒ Atezolizumab: 95% ± 7% and 98% (54%-104%) ‒ Bevacizumab: 93% ± 10% and 97% (44%-104%) ‒ Sorafenib: 84% ± 20% and 96% (27%-100%) § No specific events were responsible for the increased SAE rate in the atezolizumab + bevacizumab group § No SAEs with a ≥ 2% difference between treatment groups Finn. NEJM. 2020; 382: 1894. Grade 5 AE § Dose modification of sorafenib Slide credit: clinicaloptions. com

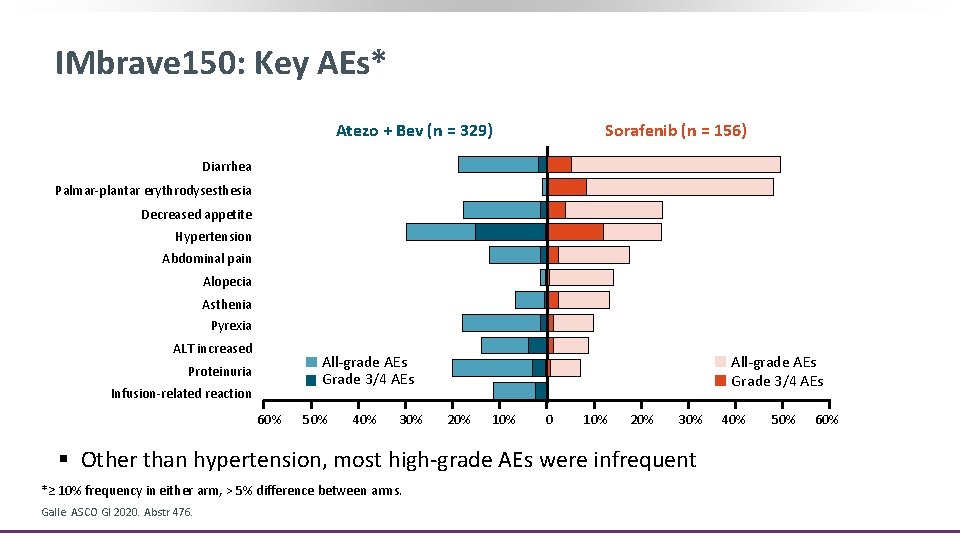
IMbrave 150: Key AEs* Atezo + Bev (n = 329) Sorafenib (n = 156) Diarrhea Palmar-plantar erythrodysesthesia Decreased appetite Hypertension Abdominal pain Alopecia Asthenia Pyrexia ALT increased All-grade AEs Grade 3/4 AEs Proteinuria Infusion-related reaction 60% 50% 40% 30% All-grade AEs Grade 3/4 AEs 20% 10% 0 10% 20% 30% § Other than hypertension, most high-grade AEs were infrequent *≥ 10% frequency in either arm, > 5% difference between arms. Galle. ASCO GI 2020. Abstr 476. 40% 50% 60%

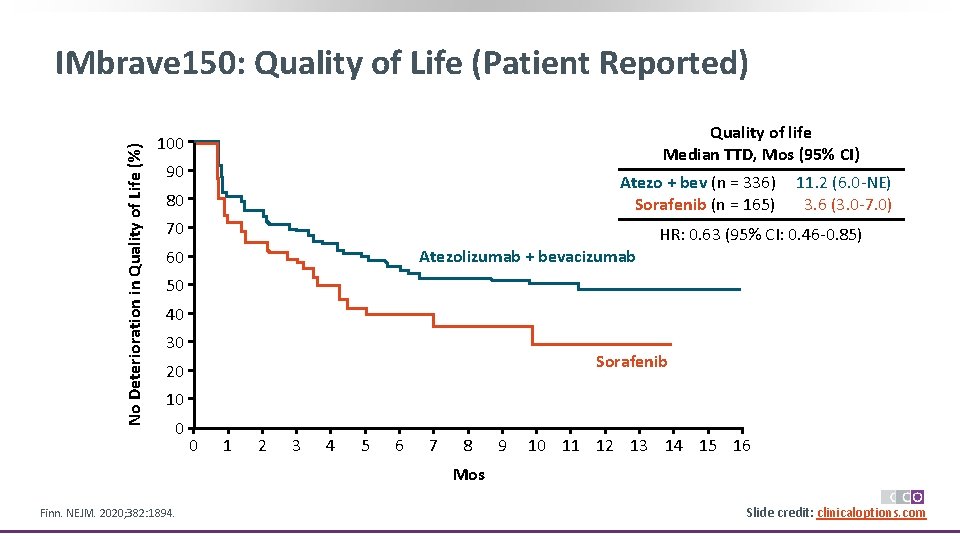
No Deterioration in Quality of Life (%) IMbrave 150: Quality of Life (Patient Reported) Quality of life Median TTD, Mos (95% CI) 100 90 Atezo + bev (n = 336) Sorafenib (n = 165) 80 70 11. 2 (6. 0 -NE) 3. 6 (3. 0 -7. 0) HR: 0. 63 (95% CI: 0. 46 -0. 85) Atezolizumab + bevacizumab 60 50 40 30 Sorafenib 20 10 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Mos Finn. NEJM. 2020; 382: 1894. Slide credit: clinicaloptions. com

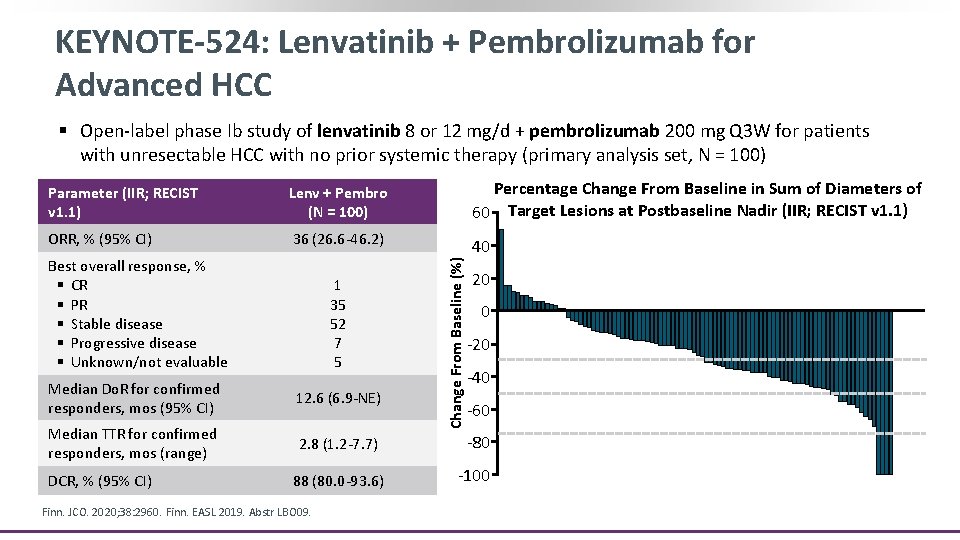
KEYNOTE-524: Lenvatinib + Pembrolizumab for Advanced HCC § Open-label phase Ib study of lenvatinib 8 or 12 mg/d + pembrolizumab 200 mg Q 3 W for patients with unresectable HCC with no prior systemic therapy (primary analysis set, N = 100) Lenv + Pembro (N = 100) Percentage Change From Baseline in Sum of Diameters of 60 Target Lesions at Postbaseline Nadir (IIR; RECIST v 1. 1) ORR, % (95% CI) 36 (26. 6 -46. 2) 40 Best overall response, % § CR § PR § Stable disease § Progressive disease § Unknown/not evaluable 1 35 52 7 5 Change From Baseline (%) Parameter (IIR; RECIST v 1. 1) 20 0 -20 -30% -40 -50% Median Do. R for confirmed responders, mos (95% CI) 12. 6 (6. 9 -NE) Median TTR for confirmed responders, mos (range) 2. 8 (1. 2 -7. 7) -80 88 (80. 0 -93. 6) -100 DCR, % (95% CI) Finn. JCO. 2020; 38: 2960. Finn. EASL 2019. Abstr LBO 09. -60 -75%

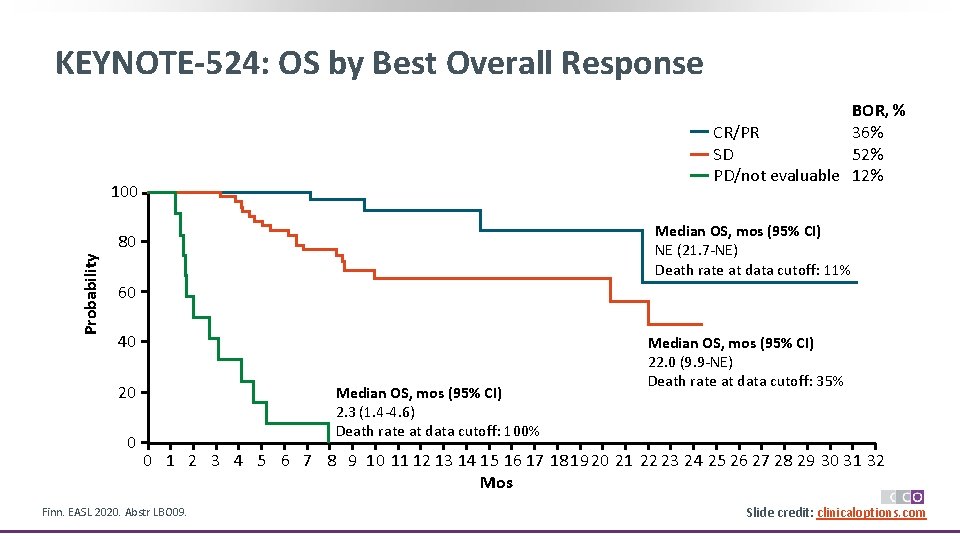
KEYNOTE-524: OS by Best Overall Response BOR, % CR/PR 36% SD 52% PD/not evaluable 12% 100 Median OS, mos (95% CI) NE (21. 7 -NE) Death rate at data cutoff: 11% Probability 80 60 40 20 0 Median OS, mos (95% CI) 2. 3 (1. 4 -4. 6) Death rate at data cutoff: 100% Median OS, mos (95% CI) 22. 0 (9. 9 -NE) Death rate at data cutoff: 35% 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 Mos Finn. EASL 2020. Abstr LBO 09. Slide credit: clinicaloptions. com

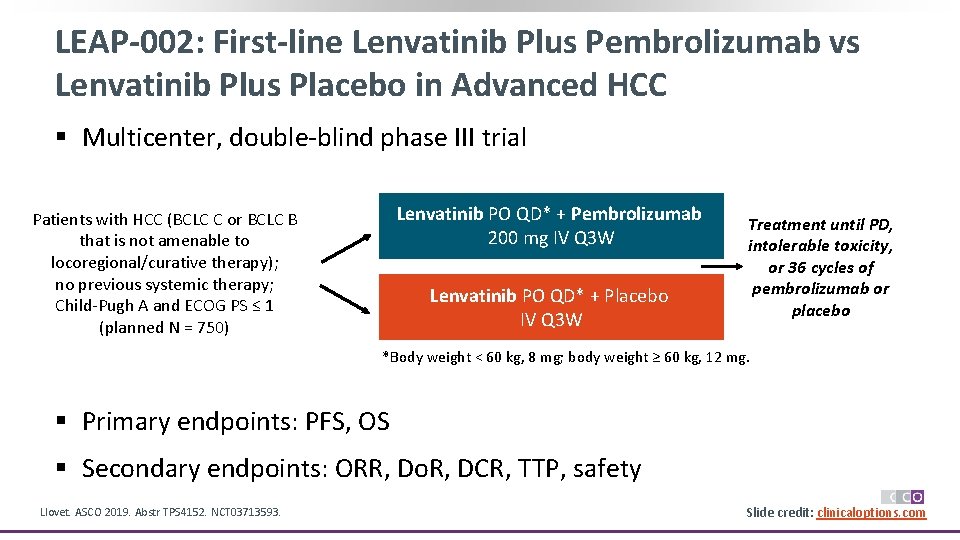
LEAP-002: First-line Lenvatinib Plus Pembrolizumab vs Lenvatinib Plus Placebo in Advanced HCC § Multicenter, double-blind phase III trial Lenvatinib PO QD* + Pembrolizumab 200 mg IV Q 3 W Patients with HCC (BCLC C or BCLC B that is not amenable to locoregional/curative therapy); no previous systemic therapy; Child-Pugh A and ECOG PS ≤ 1 (planned N = 750) Lenvatinib PO QD* + Placebo IV Q 3 W Treatment until PD, intolerable toxicity, or 36 cycles of pembrolizumab or placebo *Body weight < 60 kg, 8 mg; body weight ≥ 60 kg, 12 mg. § Primary endpoints: PFS, OS § Secondary endpoints: ORR, Do. R, DCR, TTP, safety Llovet. ASCO 2019. Abstr TPS 4152. NCT 03713593. Slide credit: clinicaloptions. com

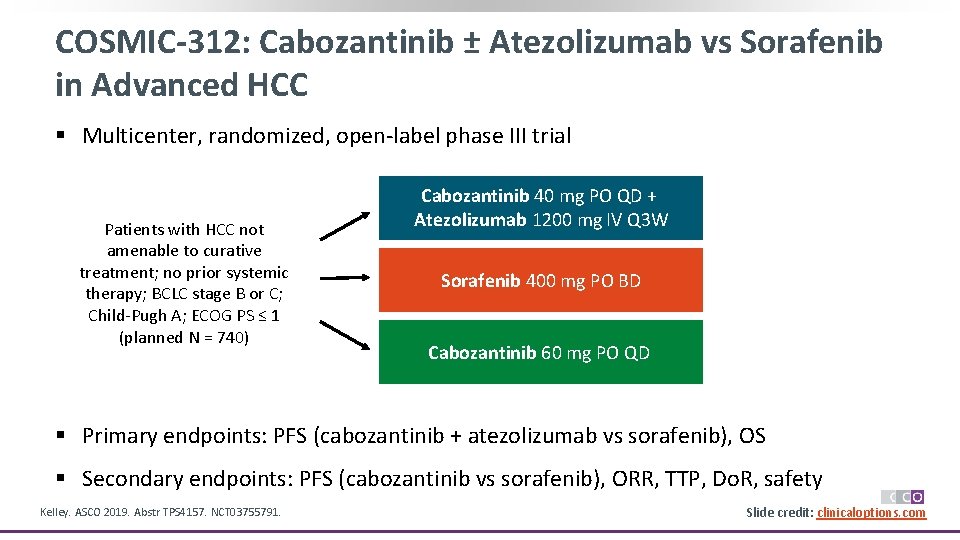
COSMIC-312: Cabozantinib ± Atezolizumab vs Sorafenib in Advanced HCC § Multicenter, randomized, open-label phase III trial Patients with HCC not amenable to curative treatment; no prior systemic therapy; BCLC stage B or C; Child-Pugh A; ECOG PS ≤ 1 (planned N = 740) Cabozantinib 40 mg PO QD + Atezolizumab 1200 mg IV Q 3 W Sorafenib 400 mg PO BD Cabozantinib 60 mg PO QD § Primary endpoints: PFS (cabozantinib + atezolizumab vs sorafenib), OS § Secondary endpoints: PFS (cabozantinib vs sorafenib), ORR, TTP, Do. R, safety Kelley. ASCO 2019. Abstr TPS 4157. NCT 03755791. Slide credit: clinicaloptions. com

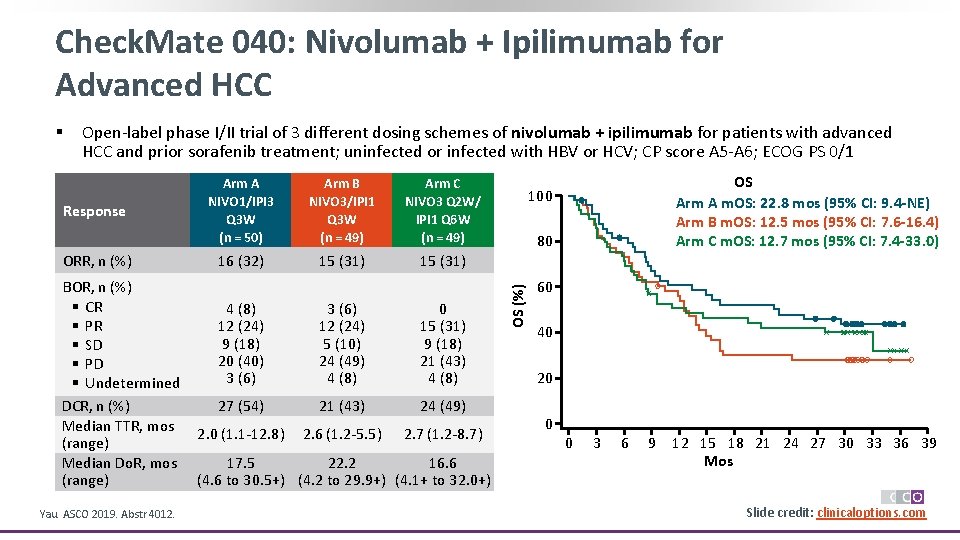
Check. Mate 040: Nivolumab + Ipilimumab for Advanced HCC Open-label phase I/II trial of 3 different dosing schemes of nivolumab + ipilimumab for patients with advanced HCC and prior sorafenib treatment; uninfected or infected with HBV or HCV; CP score A 5 -A 6; ECOG PS 0/1 Response ORR, n (%) BOR, n (%) § CR § PR § SD § PD § Undetermined DCR, n (%) Median TTR, mos (range) Median Do. R, mos (range) Yau. ASCO 2019. Abstr 4012. Arm A NIVO 1/IPI 3 Q 3 W (n = 50) Arm B NIVO 3/IPI 1 Q 3 W (n = 49) Arm C NIVO 3 Q 2 W/ IPI 1 Q 6 W (n = 49) 16 (32) 15 (31) 4 (8) 12 (24) 9 (18) 20 (40) 3 (6) 12 (24) 5 (10) 24 (49) 4 (8) 0 15 (31) 9 (18) 21 (43) 4 (8) 27 (54) 21 (43) 24 (49) 2. 0 (1. 1 -12. 8) 2. 6 (1. 2 -5. 5) 2. 7 (1. 2 -8. 7) 17. 5 22. 2 16. 6 (4. 6 to 30. 5+) (4. 2 to 29. 9+) (4. 1+ to 32. 0+) OS Arm A m. OS: 22. 8 mos (95% CI: 9. 4 -NE) Arm B m. OS: 12. 5 mos (95% CI: 7. 6 -16. 4) Arm C m. OS: 12. 7 mos (95% CI: 7. 4 -33. 0) 100 80 OS (%) § 60 40 20 0 0 3 6 9 12 15 18 21 24 27 30 33 36 39 Mos Slide credit: clinicaloptions. com

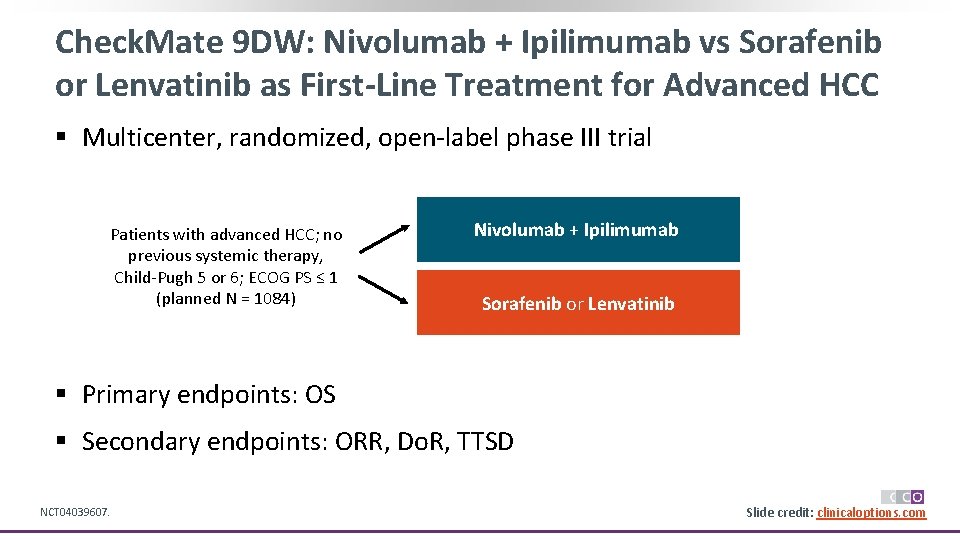
Check. Mate 9 DW: Nivolumab + Ipilimumab vs Sorafenib or Lenvatinib as First-Line Treatment for Advanced HCC § Multicenter, randomized, open-label phase III trial Patients with advanced HCC; no previous systemic therapy, Child-Pugh 5 or 6; ECOG PS ≤ 1 (planned N = 1084) Nivolumab + Ipilimumab Sorafenib or Lenvatinib § Primary endpoints: OS § Secondary endpoints: ORR, Do. R, TTSD NCT 04039607. Slide credit: clinicaloptions. com

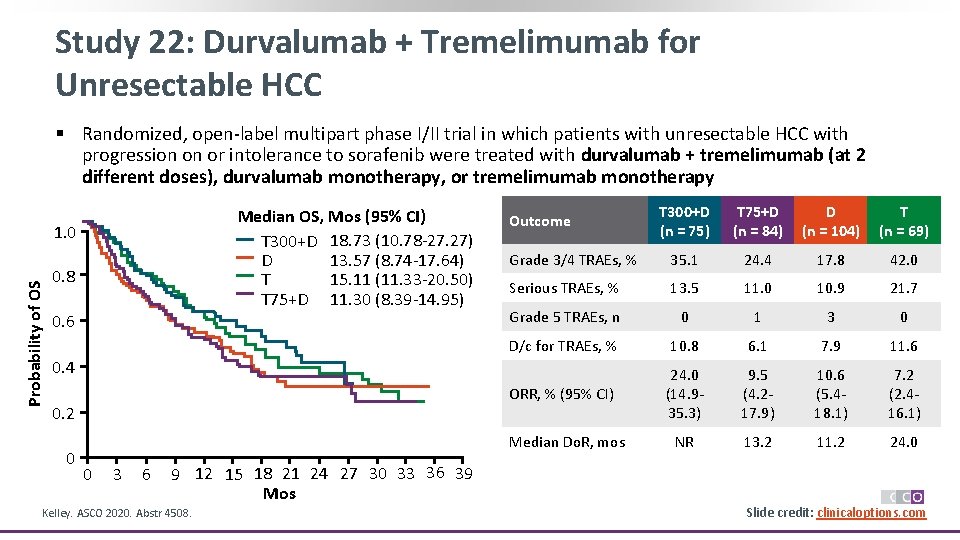
Study 22: Durvalumab + Tremelimumab for Unresectable HCC § Randomized, open-label multipart phase I/II trial in which patients with unresectable HCC with progression on or intolerance to sorafenib were treated with durvalumab + tremelimumab (at 2 different doses), durvalumab monotherapy, or tremelimumab monotherapy Median OS, Mos (95% CI) T 300+D 18. 73 (10. 78 -27. 27) 13. 57 (8. 74 -17. 64) D 15. 11 (11. 33 -20. 50) T T 75+D 11. 30 (8. 39 -14. 95) Probability of OS 1. 0 0. 8 0. 6 T 300+D (n = 75) T 75+D (n = 84) D (n = 104) T (n = 69) Grade 3/4 TRAEs, % 35. 1 24. 4 17. 8 42. 0 Serious TRAEs, % 13. 5 11. 0 10. 9 21. 7 Grade 5 TRAEs, n 0 1 3 0 D/c for TRAEs, % 10. 8 6. 1 7. 9 11. 6 ORR, % (95% CI) 24. 0 (14. 935. 3) 9. 5 (4. 217. 9) 10. 6 (5. 418. 1) 7. 2 (2. 416. 1) NR 13. 2 11. 2 24. 0 Outcome 0. 4 0. 2 0 Median Do. R, mos 0 3 6 9 12 15 18 21 24 27 30 33 36 39 Mos Kelley. ASCO 2020. Abstr 4508. Slide credit: clinicaloptions. com

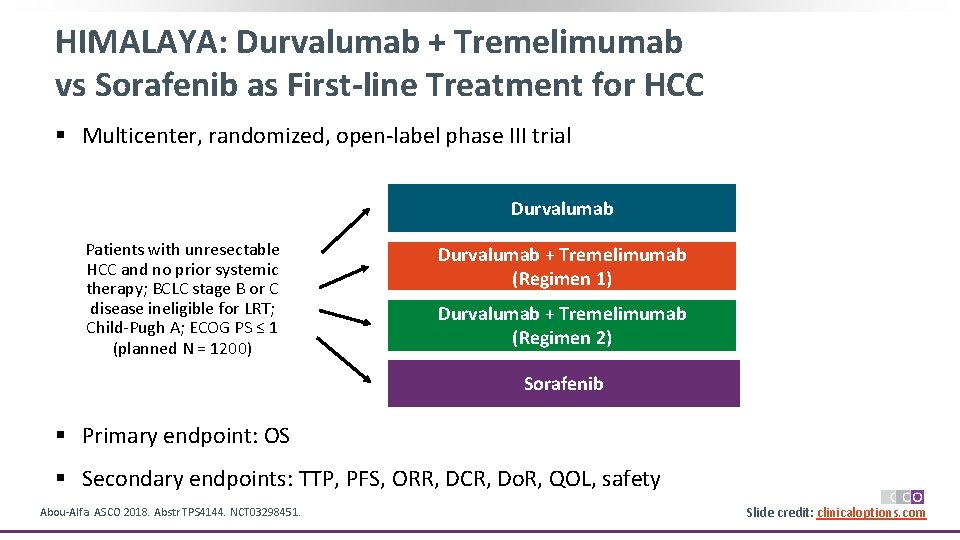
HIMALAYA: Durvalumab + Tremelimumab vs Sorafenib as First-line Treatment for HCC § Multicenter, randomized, open-label phase III trial Durvalumab Patients with unresectable HCC and no prior systemic therapy; BCLC stage B or C disease ineligible for LRT; Child-Pugh A; ECOG PS ≤ 1 (planned N = 1200) Durvalumab + Tremelimumab (Regimen 1) Durvalumab + Tremelimumab (Regimen 2) Sorafenib § Primary endpoint: OS § Secondary endpoints: TTP, PFS, ORR, DCR, Do. R, QOL, safety Abou-Alfa. ASCO 2018. Abstr TPS 4144. NCT 03298451. Slide credit: clinicaloptions. com

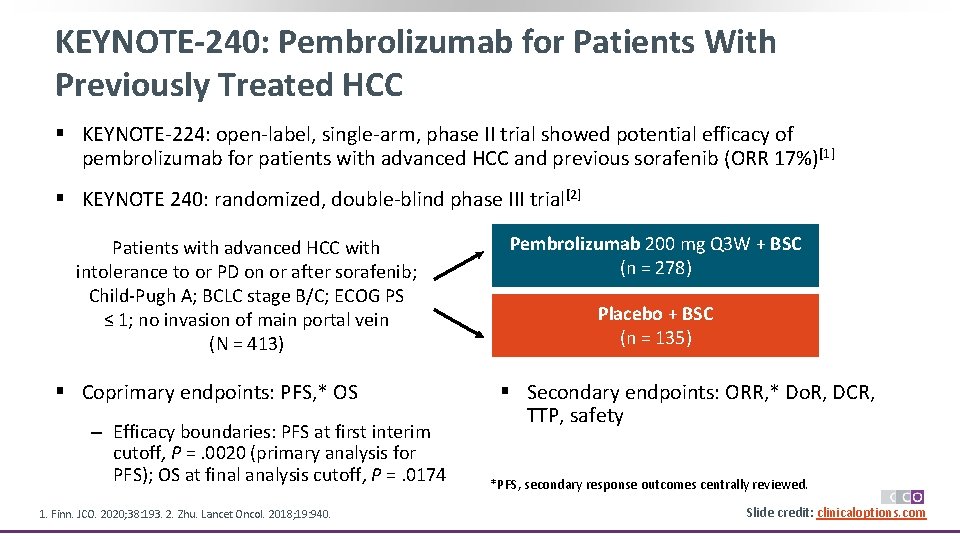
KEYNOTE-240: Pembrolizumab for Patients With Previously Treated HCC § KEYNOTE-224: open-label, single-arm, phase II trial showed potential efficacy of pembrolizumab for patients with advanced HCC and previous sorafenib (ORR 17%)[1] § KEYNOTE 240: randomized, double-blind phase III trial[2] Patients with advanced HCC with intolerance to or PD on or after sorafenib; Child-Pugh A; BCLC stage B/C; ECOG PS ≤ 1; no invasion of main portal vein (N = 413) § Coprimary endpoints: PFS, * OS ‒ Efficacy boundaries: PFS at first interim cutoff, P =. 0020 (primary analysis for PFS); OS at final analysis cutoff, P =. 0174 1. Finn. JCO. 2020; 38: 193. 2. Zhu. Lancet Oncol. 2018; 19: 940. Pembrolizumab 200 mg Q 3 W + BSC (n = 278) Placebo + BSC (n = 135) § Secondary endpoints: ORR, * Do. R, DCR, TTP, safety *PFS, secondary response outcomes centrally reviewed. Slide credit: clinicaloptions. com

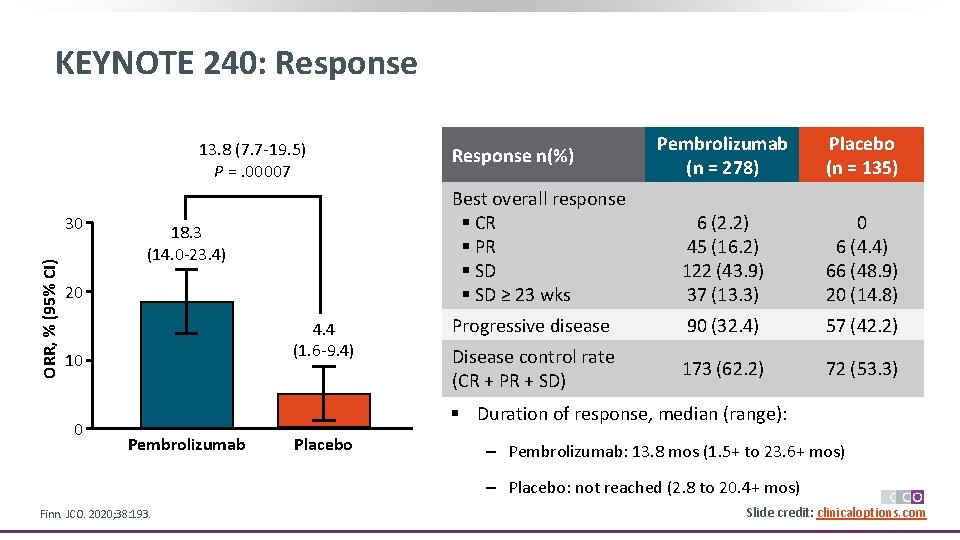
KEYNOTE 240: Response 13. 8 (7. 7 -19. 5) 13. 8 (7. 7 – 19. 5) P =. 00007 p = 0. 00007 a ORR, % (95% CI) 30 18. 3 (14. 0 -23. 4) 20 4. 4 (1. 6 -9. 4) 10 0 Pembrolizumab (n = 278) Placebo (n = 135) Best overall response § CR § PR § SD ≥ 23 wks 6 (2. 2) 45 (16. 2) 122 (43. 9) 37 (13. 3) 0 6 (4. 4) 66 (48. 9) 20 (14. 8) Progressive disease 90 (32. 4) 57 (42. 2) Disease control rate (CR + PR + SD) 173 (62. 2) 72 (53. 3) Response n(%) § Duration of response, median (range): Pembrolizumab Placebo ‒ Pembrolizumab: 13. 8 mos (1. 5+ to 23. 6+ mos) ‒ Placebo: not reached (2. 8 to 20. 4+ mos) Finn. JCO. 2020; 38: 193. Slide credit: clinicaloptions. com

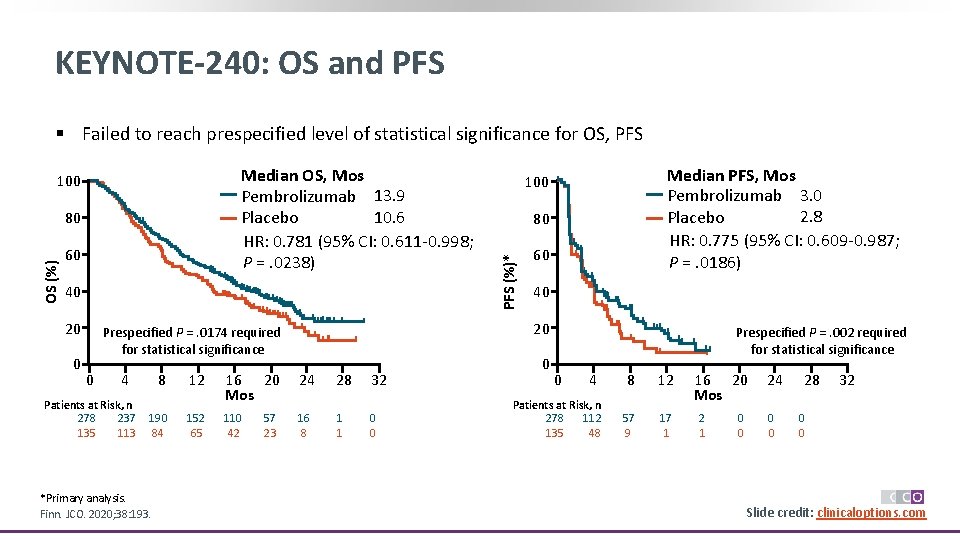
KEYNOTE-240: OS and PFS § Failed to reach prespecified level of statistical significance for OS, PFS OS (%) 80 60 40 20 0 4 Patients at Risk, n 278 237 135 113 8 12 16 20 Mos 24 28 32 190 84 152 65 110 42 16 8 1 1 0 0 *Primary analysis. Finn. JCO. 2020; 38: 193. 57 23 80 60 40 20 Prespecified P =. 0174 required for statistical significance 0 Median PFS, Mos Pembrolizumab 3. 0 2. 8 Placebo HR: 0. 775 (95% CI: 0. 609 -0. 987; P =. 0186) 100 PFS (%)* Median OS, Mos Pembrolizumab 13. 9 10. 6 Placebo HR: 0. 781 (95% CI: 0. 611 -0. 998; P =. 0238) 100 0 Prespecified P =. 002 required for statistical significance 0 4 Patients at Risk, n 278 112 135 48 8 12 57 9 17 1 16 20 Mos 2 1 0 0 24 0 0 28 32 0 0 Slide credit: clinicaloptions. com

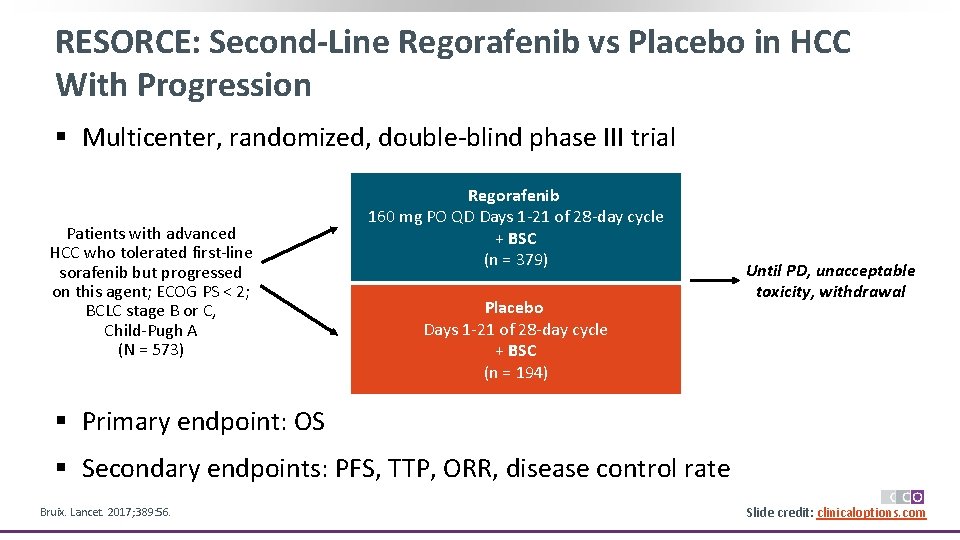
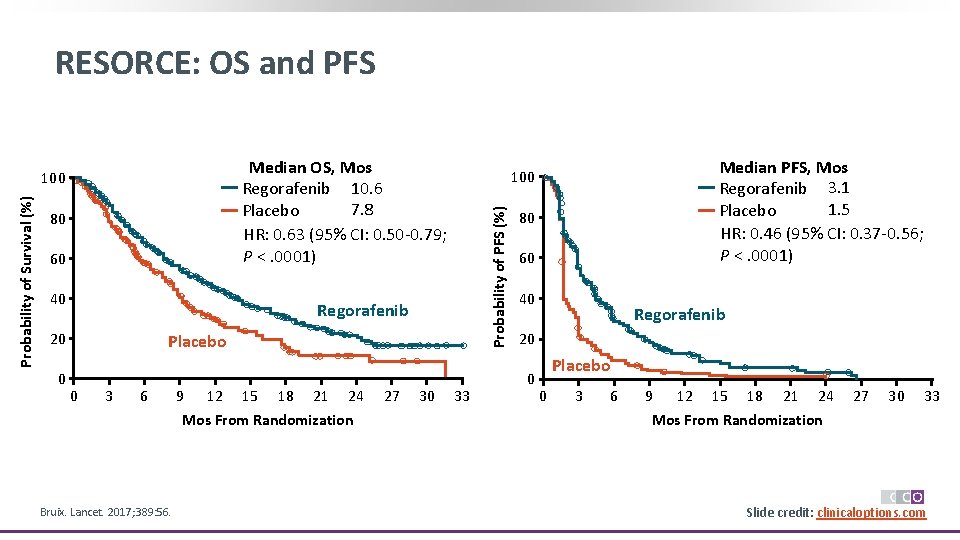
RESORCE: Second-Line Regorafenib vs Placebo in HCC With Progression § Multicenter, randomized, double-blind phase III trial Patients with advanced HCC who tolerated first-line sorafenib but progressed on this agent; ECOG PS < 2; BCLC stage B or C, Child-Pugh A (N = 573) Regorafenib 160 mg PO QD Days 1 -21 of 28 -day cycle + BSC (n = 379) Placebo Days 1 -21 of 28 -day cycle + BSC (n = 194) Until PD, unacceptable toxicity, withdrawal § Primary endpoint: OS § Secondary endpoints: PFS, TTP, ORR, disease control rate Bruix. Lancet. 2017; 389: 56. Slide credit: clinicaloptions. com

RESORCE: OS and PFS Median OS, Mos Regorafenib 10. 6 7. 8 Placebo HR: 0. 63 (95% CI: 0. 50 -0. 79; P <. 0001) 80 60 40 Regorafenib 20 0 Placebo 0 3 6 9 12 15 18 21 24 Mos From Randomization Bruix. Lancet. 2017; 389: 56. 27 30 Median PFS, Mos Regorafenib 3. 1 1. 5 Placebo HR: 0. 46 (95% CI: 0. 37 -0. 56; P <. 0001) 100 Probability of PFS (%) Probability of Survival (%) 100 33 80 60 40 Regorafenib 20 0 Placebo 0 3 6 9 12 15 18 21 24 27 30 33 Mos From Randomization Slide credit: clinicaloptions. com

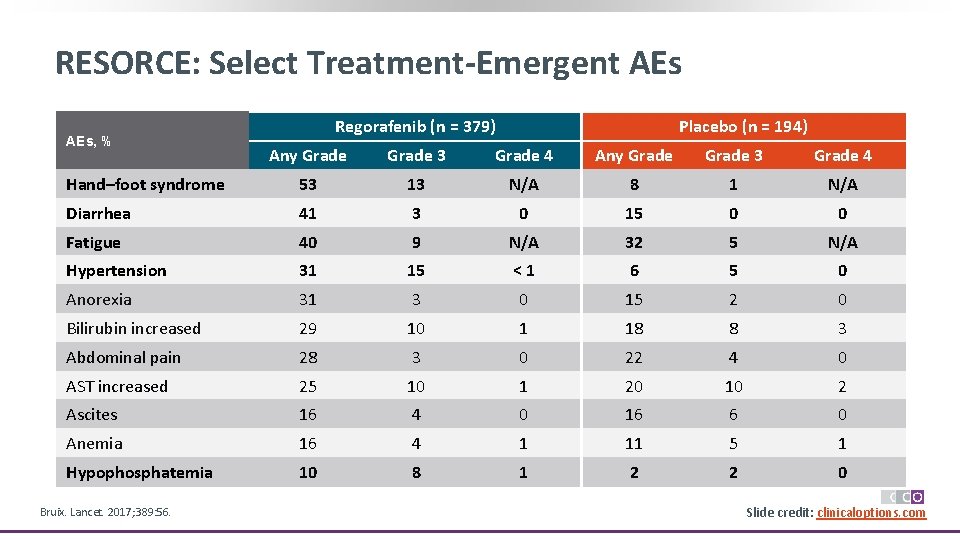
RESORCE: Select Treatment-Emergent AEs, % Regorafenib (n = 379) Placebo (n = 194) Any Grade 3 Grade 4 Hand–foot syndrome 53 13 N/A 8 1 N/A Diarrhea 41 3 0 15 0 0 Fatigue 40 9 N/A 32 5 N/A Hypertension 31 15 <1 6 5 0 Anorexia 31 3 0 15 2 0 Bilirubin increased 29 10 1 18 8 3 Abdominal pain 28 3 0 22 4 0 AST increased 25 10 1 20 10 2 Ascites 16 4 0 16 6 0 Anemia 16 4 1 11 5 1 Hypophosphatemia 10 8 1 2 2 0 Bruix. Lancet. 2017; 389: 56. Slide credit: clinicaloptions. com

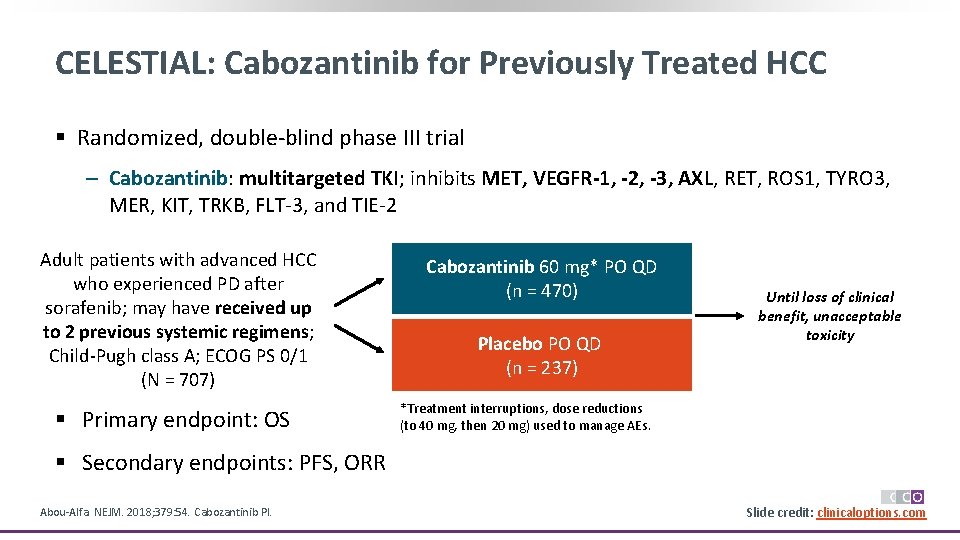
CELESTIAL: Cabozantinib for Previously Treated HCC § Randomized, double-blind phase III trial ‒ Cabozantinib: multitargeted TKI; inhibits MET, VEGFR-1, -2, -3, AXL, RET, ROS 1, TYRO 3, MER, KIT, TRKB, FLT-3, and TIE-2 Adult patients with advanced HCC who experienced PD after sorafenib; may have received up to 2 previous systemic regimens; Child-Pugh class A; ECOG PS 0/1 (N = 707) § Primary endpoint: OS Cabozantinib 60 mg* PO QD (n = 470) Placebo PO QD (n = 237) Until loss of clinical benefit, unacceptable toxicity *Treatment interruptions, dose reductions (to 40 mg, then 20 mg) used to manage AEs. § Secondary endpoints: PFS, ORR Abou-Alfa. NEJM. 2018; 379: 54. Cabozantinib PI. Slide credit: clinicaloptions. com

CELESTIAL: OS and PFS Probability of OS 1. 0 0. 8 0. 6 Cabozantinib 0. 4 0. 2 0 Placebo 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 Mos Abou-Alfa. NEJM. 2018; 379: 54. Median PFS, Mos Cabozantinib 5. 2 1. 9 Placebo HR: 0. 44 (95% CI: 0. 36 -0. 52; P <. 001) 1. 0 Probability of PFS Median OS, Mos Cabozantinib 10. 2 8. 0 Placebo HR: 0. 76 (95% CI: 0. 63 -0. 92; P =. 005) 0. 8 0. 6 Cabozantinib 0. 4 0. 2 0 Placebo 0 3 6 9 12 15 18 21 24 Mos Slide credit: clinicaloptions. com

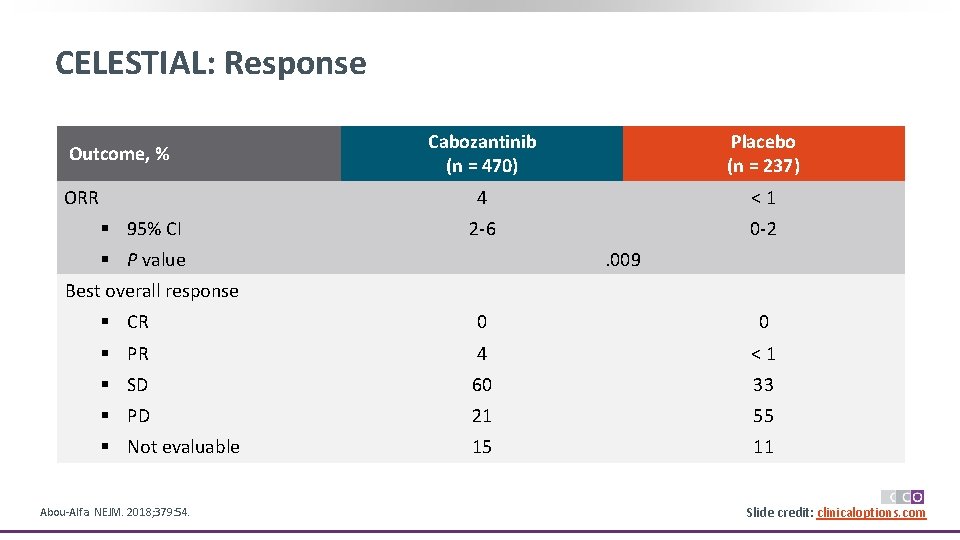
CELESTIAL: Response Outcome, % ORR § 95% CI Cabozantinib (n = 470) Placebo (n = 237) 4 <1 2 -6 0 -2 § P value . 009 Best overall response § CR 0 0 § PR 4 <1 § SD 60 33 § PD 21 55 § Not evaluable 15 11 Abou-Alfa. NEJM. 2018; 379: 54. Slide credit: clinicaloptions. com

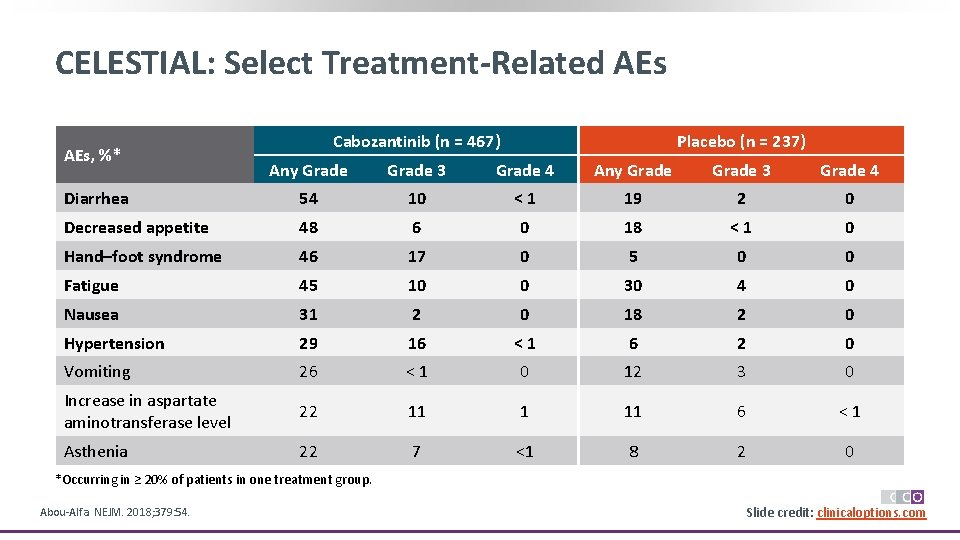
CELESTIAL: Select Treatment-Related AEs, %* Cabozantinib (n = 467) Placebo (n = 237) Any Grade 3 Grade 4 Diarrhea 54 10 <1 19 2 0 Decreased appetite 48 6 0 18 <1 0 Hand–foot syndrome 46 17 0 5 0 0 Fatigue 45 10 0 30 4 0 Nausea 31 2 0 18 2 0 Hypertension 29 16 <1 6 2 0 Vomiting 26 <1 0 12 3 0 Increase in aspartate aminotransferase level 22 11 1 11 6 <1 Asthenia 22 7 <1 8 2 0 *Occurring in ≥ 20% of patients in one treatment group. Abou-Alfa. NEJM. 2018; 379: 54. Slide credit: clinicaloptions. com

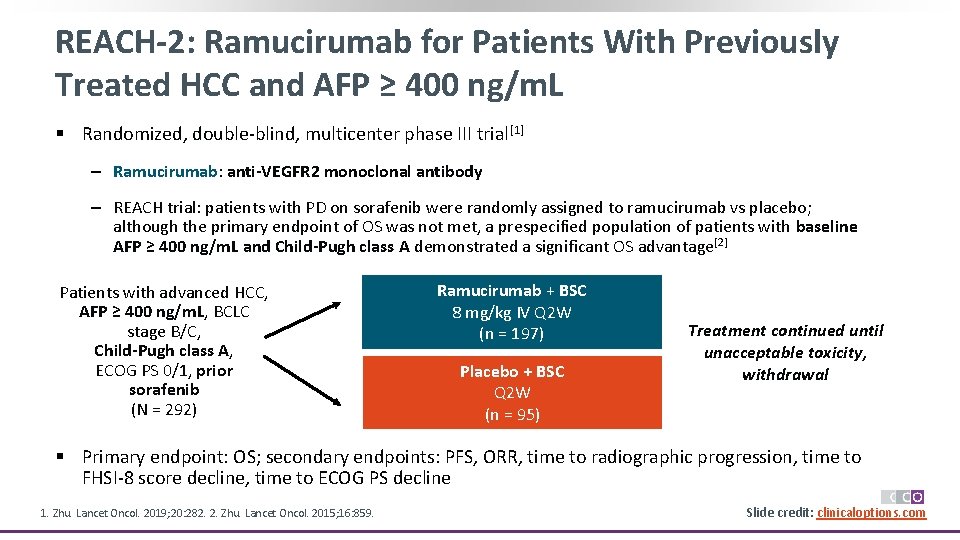
REACH-2: Ramucirumab for Patients With Previously Treated HCC and AFP ≥ 400 ng/m. L § Randomized, double-blind, multicenter phase III trial[1] ‒ Ramucirumab: anti-VEGFR 2 monoclonal antibody ‒ REACH trial: patients with PD on sorafenib were randomly assigned to ramucirumab vs placebo; although the primary endpoint of OS was not met, a prespecified population of patients with baseline AFP ≥ 400 ng/m. L and Child-Pugh class A demonstrated a significant OS advantage[2] Patients with advanced HCC, AFP ≥ 400 ng/m. L, BCLC stage B/C, Child-Pugh class A, ECOG PS 0/1, prior sorafenib (N = 292) Ramucirumab + BSC 8 mg/kg IV Q 2 W (n = 197) Placebo + BSC Q 2 W (n = 95) Treatment continued until unacceptable toxicity, withdrawal § Primary endpoint: OS; secondary endpoints: PFS, ORR, time to radiographic progression, time to FHSI-8 score decline, time to ECOG PS decline 1. Zhu. Lancet Oncol. 2019; 20: 282. 2. Zhu. Lancet Oncol. 2015; 16: 859. Slide credit: clinicaloptions. com

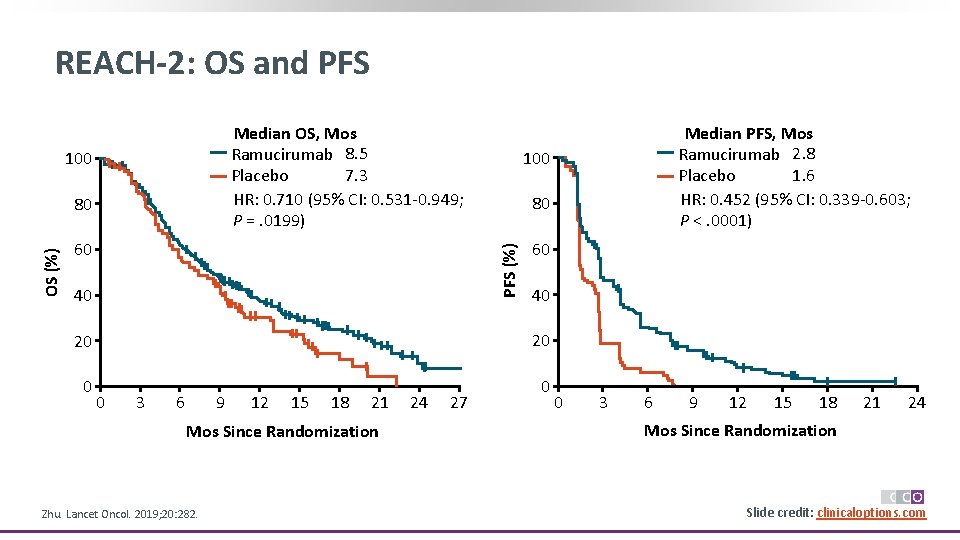
REACH-2: OS and PFS Median OS, Mos Ramucirumab 8. 5 7. 3 Placebo HR: 0. 710 (95% CI: 0. 531 -0. 949; P =. 0199) 100 60 100 80 PFS (%) OS (%) 80 40 60 40 20 20 0 3 6 9 12 15 18 21 Mos Since Randomization Zhu. Lancet Oncol. 2019; 20: 282. 24 27 Median PFS, Mos Ramucirumab 2. 8 1. 6 Placebo HR: 0. 452 (95% CI: 0. 339 -0. 603; P <. 0001) 0 3 6 9 12 15 18 21 24 Mos Since Randomization Slide credit: clinicaloptions. com

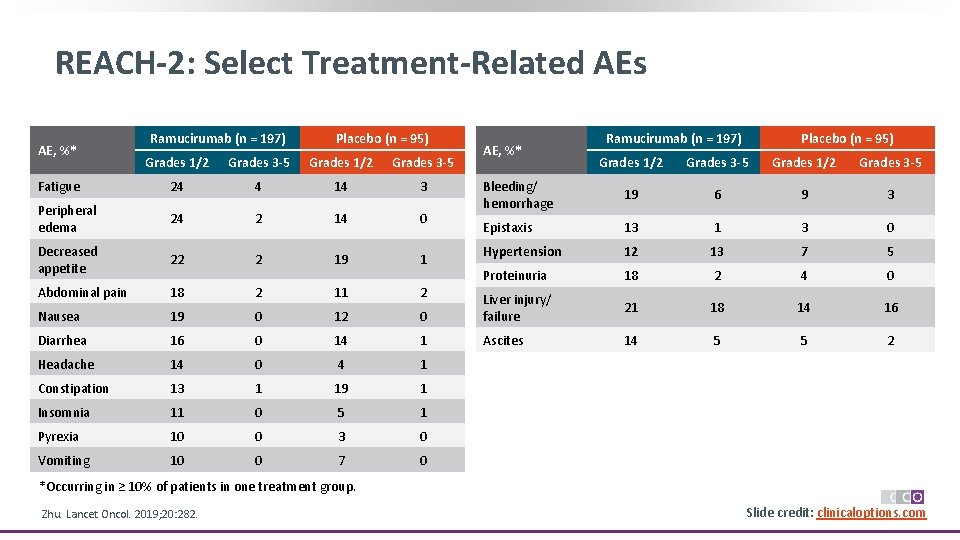
REACH-2: Select Treatment-Related AEs AE, %* Ramucirumab (n = 197) Placebo (n = 95) Grades 1/2 Grades 3 -5 Fatigue 24 4 14 3 Peripheral edema 24 2 14 0 Decreased appetite 22 2 19 1 Abdominal pain 18 2 11 2 Nausea 19 0 12 Diarrhea 16 0 Headache 14 Constipation AE, %* Ramucirumab (n = 197) Placebo (n = 95) Grades 1/2 Grades 3 -5 Bleeding/ hemorrhage 19 6 9 3 Epistaxis 13 1 3 0 Hypertension 12 13 7 5 Proteinuria 18 2 4 0 0 Liver injury/ failure 21 18 14 16 14 1 Ascites 14 5 5 2 0 4 1 13 1 19 1 Insomnia 11 0 5 1 Pyrexia 10 0 3 0 Vomiting 10 0 7 0 *Occurring in ≥ 10% of patients in one treatment group. Zhu. Lancet Oncol. 2019; 20: 282. Slide credit: clinicaloptions. com

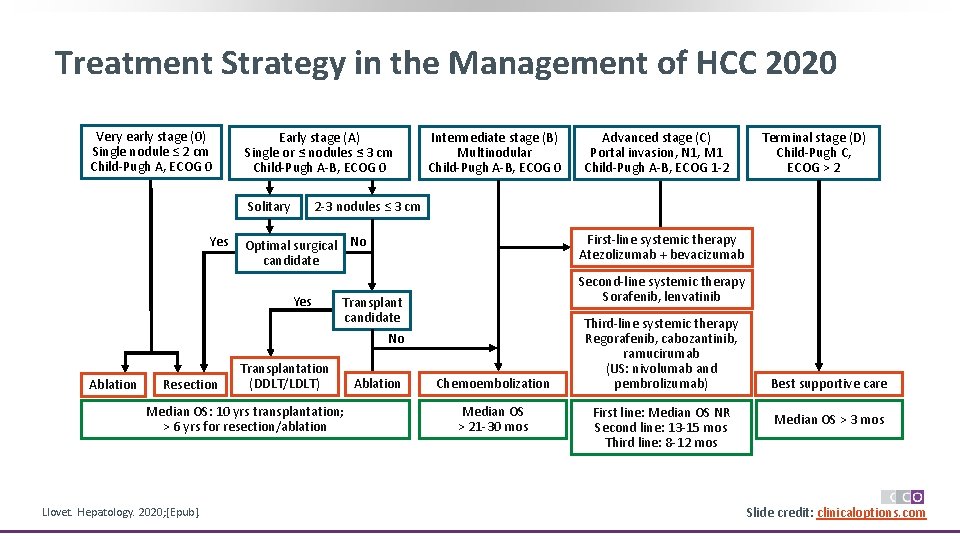
Treatment Strategy in the Management of HCC 2020 Very early stage (0) Single nodule ≤ 2 cm Child-Pugh A, ECOG 0 Early stage (A) Single or ≤ nodules ≤ 3 cm Child-Pugh A-B, ECOG 0 Solitary Yes Ablation Resection Advanced stage (C) Portal invasion, N 1, M 1 Child-Pugh A-B, ECOG 1 -2 Terminal stage (D) Child-Pugh C, ECOG > 2 2 -3 nodules ≤ 3 cm Optimal surgical No candidate First-line systemic therapy Atezolizumab + bevacizumab Yes Second-line systemic therapy Sorafenib, lenvatinib Transplant candidate No Transplantation (DDLT/LDLT) Median OS: 10 yrs transplantation; > 6 yrs for resection/ablation Llovet. Hepatology. 2020; [Epub]. Intermediate stage (B) Multinodular Child-Pugh A-B, ECOG 0 Ablation Chemoembolization Median OS > 21 -30 mos Third-line systemic therapy Regorafenib, cabozantinib, ramucirumab (US: nivolumab and pembrolizumab) First line: Median OS NR Second line: 13 -15 mos Third line: 8 -12 mos Best supportive care Median OS > 3 mos Slide credit: clinicaloptions. com

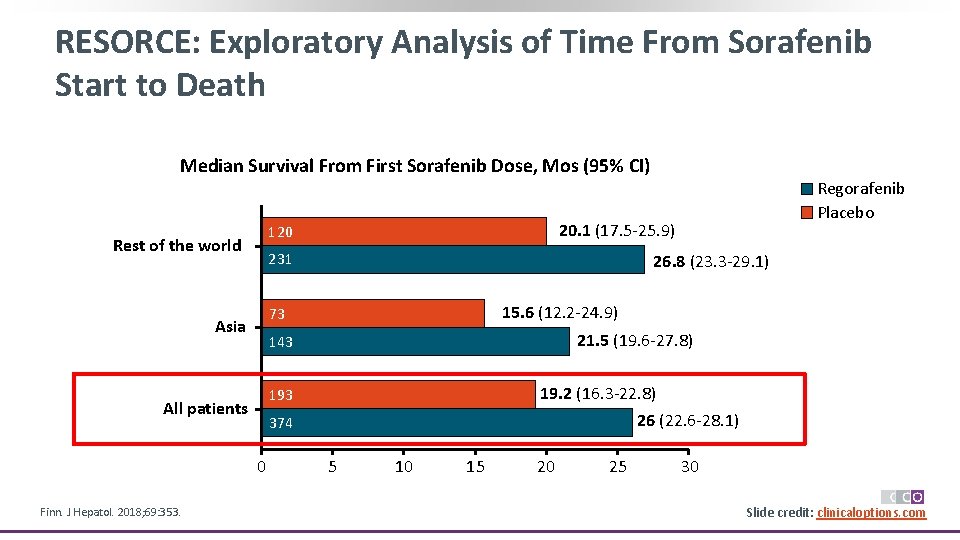
RESORCE: Exploratory Analysis of Time From Sorafenib Start to Death Median Survival From First Sorafenib Dose, Mos (95% CI) 231 26. 8 (23. 3 -29. 1) 15. 6 (12. 2 -24. 9) 73 Asia All patients 143 21. 5 (19. 6 -27. 8) 193 19. 2 (16. 3 -22. 8) 26 (22. 6 -28. 1) 374 0 Finn. J Hepatol. 2018; 69: 353. 20. 1 (17. 5 -25. 9) 120 Rest of the world Regorafenib Placebo 5 10 15 20 25 30 Slide credit: clinicaloptions. com

Conclusions § After nearly a decade, we have had 5 positive phase III studies of new drugs in HCC that improve OS ‒ Atezolizumab plus bevacizumab: first line, superior to sorafenib (HR: 0. 58) ‒ Lenvatinib: first line, noninferior to sorafenib (HR: 0. 92) ‒ Regorafenib: second line (post sorafenib), superior to placebo (HR: 0. 63) ‒ Cabozantinib: second line (post sorafenib) and third line, superior to placebo (HR: 0. 70 when prior sorafenib) ‒ Ramucirumab: second line (post sorafenib) when high AFP, superior to placebo (HR: 0. 71) § For the first time, we have a highly active regimen that is superior to sorafenib as frontline therapy (atezolizumab plus bevacizumab) § Level 1 evidence for single-agent immune checkpoint inhibitors still lacking § Ongoing studies looking at novel combinations ‒ Immune checkpoint inhibitors plus TKIs ‒ PD-1 inhibitor plus CTLA-4 inhibitor

Go Online for More CCO Coverage of HCC! Downloadable slides with all the key data from this presentation Downloadable resource summarizing key concepts in optimizing treatment for patients with advanced HCC Interactive Treatment Decision Support Tool for HCC Enter your own case scenarios to get management recommendations from 5 HCC experts (coming soon) clinicaloptions. com/oncology