Construction of Gateway TMbased vectors for highthroughput cloning
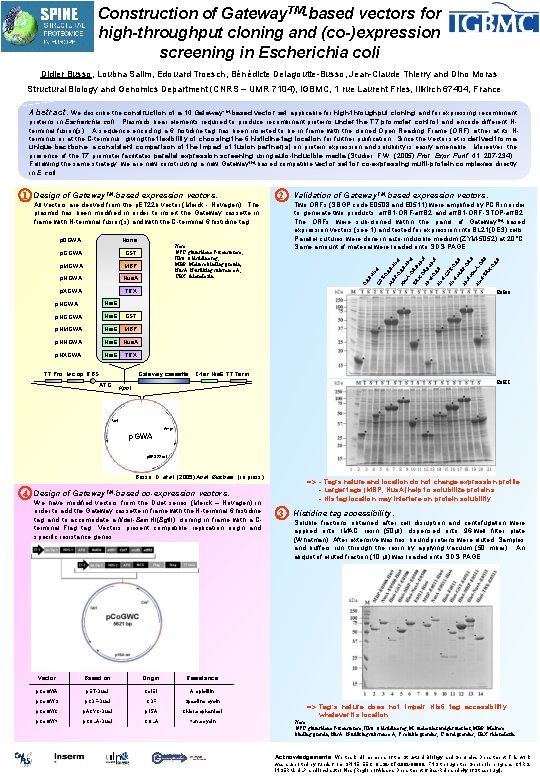
Construction of Gateway. TM-based vectors for high-throughput cloning and (co-)expression screening in Escherichia coli Didier Busso, Loubna Salim, Edouard Troesch, Bénédicte Delagoutte-Busso, Jean-Claude Thierry and Dino Moras Structural Biology and Genomics Department (CNRS – UMR 7104), IGBMC, 1 rue Laurent Fries, Illkirch 67404, France Abstract. We describe the construction of a 10 Gateway. TM-based vector set applicable for high-throughput cloning and for expressing recombinant proteins in Escherichia coli. Plasmids bear elements required to produce recombinant proteins under the T 7 promoter control and encode different Nterminal fusion(s). A sequence encoding a 6 histidine tag has been inserted to be in frame with the cloned Open Reading Frame (ORF) either at its Nterminus or at the C-terminus, giving the flexibility of choosing the 6 histidine tag location for further purification. Since the vector set is derived from a unique backbone, a consistent comparison of the impact of fusion partner(s) on protein expression and solubility is easily amenable. Moreover, the presence of the T 7 promoter facilitates parallel expression screening using auto-inducible media (Studier, F. W. (2005) Prot. Expr. Purif. 41: 207 -234). Following the same strategy, we are now constructing a new Gateway. TM-based compatible vector set for co-expressing multi-protein complexes directly in E. coli. p. HGWA His 6 p. HGGWA His 6 GST p. HMGWA His 6 MBP p. HNGWA His 6 Nus. A p. HXGWA His 6 RF OR -N F us AHi OR s 6 -T F RX -O RF -O BP - -M s 6 RF ST -G s 6 Hi Hi Hi s 6 Hi F- -O Hi s 6 Hi F- OR X- -O R TR s 6 Hi Hi F- E 0508 TRX T 7 Pro lac op RBS Gateway cassette ATG s. A F- TRX p. XGWA OR Nus. A BP - p. NGWA Nu MBP s 6 p. MGWA Note: GST: glutathione S-transferase, His 6: 6 histidine tag, MBP: Maltose binding protein, Nus. A: N-utilizing substance A, TRX: thioredoxin. OR GST Two ORFs (SBGP code E 0508 and E 0511) were amplified by PCR in order to generate two products: att. B 1 -ORF-att. B 2 and att. B 1 -ORF-STOP-att. B 2. The ORFs were sub-cloned within the panel of Gateway. TM-based expression vectors (see 1) and tested for expression into BL 21(DE 3) cells. Parallel cultures were done in auto-inducible medium (ZYM-5052) at 20°C. Same amount of material were loaded onto SDS-PAGE. FHi None p. GGWA Validation of Gateway. TM-based expression vectors. OR p 0 GWA 2 M All vectors are derived from the p. ET 22 b vector (Merck - Novagen). The plasmid has been modified in order to insert the Gateway cassette in frame with N-terminal fusion(s) and with the C-terminal 6 histidine tag. T- Design of Gateway. TM-based expression vectors. GS 1 C-ter His 6 T 7 Term. E 0511 Kpn. I lac. I Ampr p. GWA p. BR 322 ori Busso, D. et al. (2005) Anal. Biochem. (in press) 4 Design of Gateway TM-based => - Tag’s nature and location do not change expression profile. - Larger tags (MBP, Nus. A) help to solubilize proteins. - His tag location may interfere on protein solubility. co-expression vectors. We have modified vectors from the Duet series (Merck – Novagen) in order to add the Gateway cassette in frame with the N-terminal 6 histidine tag and to accomodate a Nde. I-Bam. HI(Bgl. II) cloning in frame with a Cterminal Flag tag. Vectors present compatible replication origin and specific resistance genes. Vector Based on Origin p. Co. GWA p. ET-Duet Col. E 1 Ampicillin p. Co. GWS p. CDF-Duet CDF Spectinomycin p. Co. GWC p. ACYC-Duet p 15 A Chloramphenicol p. Co. GWK p. COLA-Duet COLA Kanamycin 3 Histidine tag accessibility. Soluble fractions obtained after cell disruption and centrifugation were applied onto IMAC resin (50µl) dispensed onto 96 -well filter plate (Whatman). After extensive washes, bound proteins were eluted. Samples and buffers run through the resin by applying vacuum (50 mbar). An aliquot of eluted fraction (10 µl) was loaded onto SDS-PAGE. Resistance => Tag’s nature does not impair His 6 tag accessibility whatever its location. Note: GST: glutathione S-transferase, His 6: 6 histidine tag, M: molecular weight marker, MBP: Maltose binding protein, Nus. A: N-utilizing substance A, S: soluble proteins, T: total proteins, TRX: thioredoxin. Acknowledgements: We thank all members of the Structural Biology and Genomics Department. This work was supported by funds from SPINE EEC QLG 2 -CT-2002 -00988, FNS through the Genopole program, CNRS, INSERM, ULP and local authorities (Region of Alsace, Department of Bas-Rhin and city of Strasbourg).
- Slides: 1