Constructing Vertical Sections A Five Step Method Click


- Slides: 9

Constructing Vertical Sections A Five Step Method Click to continue

The Five Steps: • • • Click to continue Solidus Line Liquidus Line Sub-Liquidus Line Alchemades Line Intersections Labels

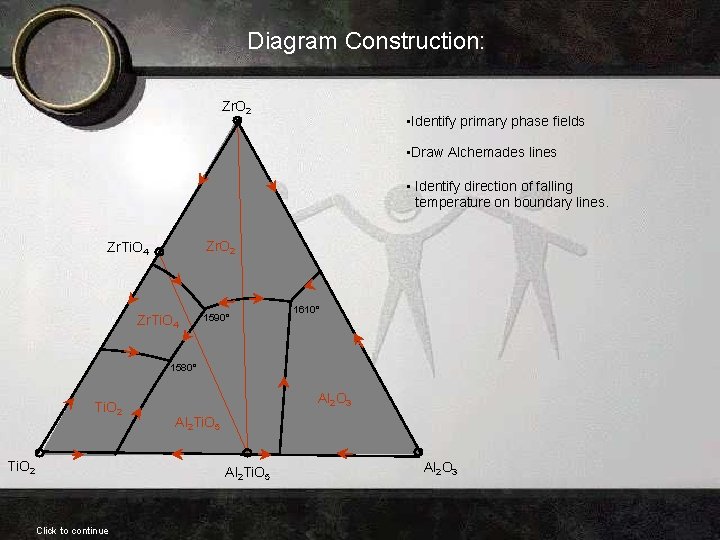
Diagram Construction: Zr. O 2 • Identify primary phase fields • Draw Alchemades lines • Identify direction of falling temperature on boundary lines. Zr. O 2 Zr. Ti. O 4 1590° 1610° 1580° Ti. O 2 Al 2 O 3 Al 2 Ti. O 5 Click to continue Al 2 O 3

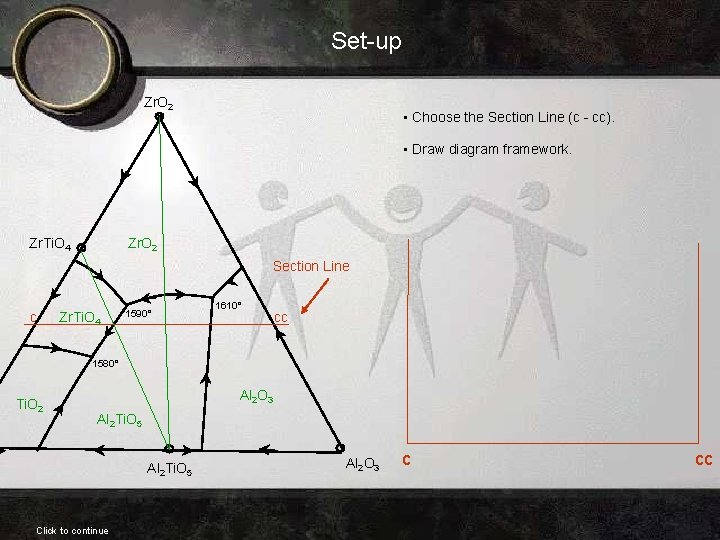
Set-up Zr. O 2 • Choose the Section Line (c - cc). • Draw diagram framework. Zr. O 2 Zr. Ti. O 4 Section Line c Zr. Ti. O 4 1590° 1610° cc 1580° Ti. O 2 Al 2 O 3 Al 2 Ti. O 5 Click to continue Al 2 O 3 c cc

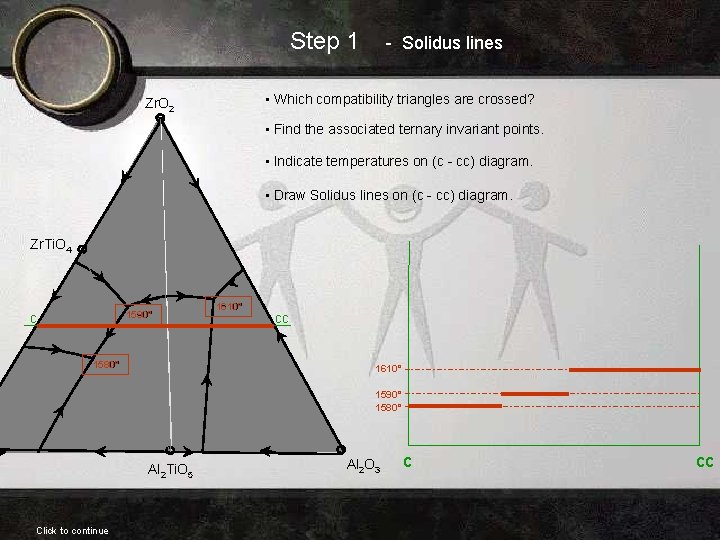
Step 1 - Solidus lines • Which compatibility triangles are crossed? Zr. O 2 • Find the associated ternary invariant points. • Indicate temperatures on (c - cc) diagram. • Draw Solidus lines on (c - cc) diagram. Zr. Ti. O 4 c Zr. O 2 Zr. Ti. O 4 1590° 1610° 1580° Ti. O 2 1610° Al 2 O 3 Al 2 Ti. O 5 Click to continue cc 1590° 1580° Al 2 O 3 c cc

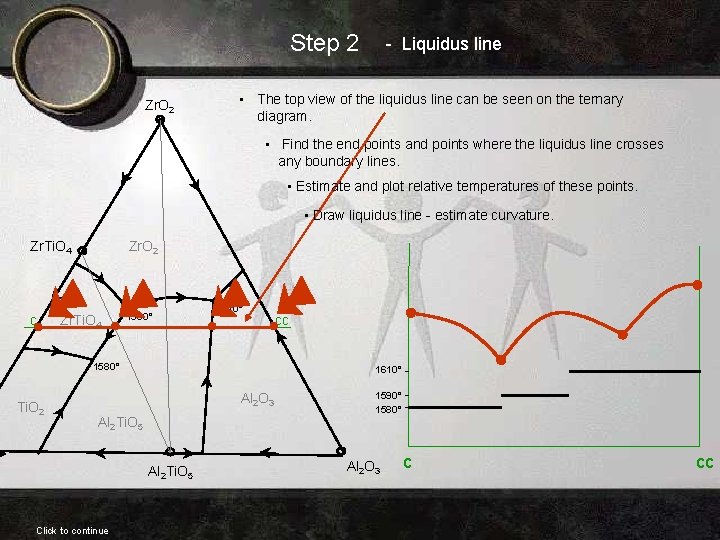
Step 2 Zr. O 2 - Liquidus line • The top view of the liquidus line can be seen on the ternary diagram. • Find the end points and points where the liquidus line crosses any boundary lines. • Estimate and plot relative temperatures of these points. • Draw liquidus line - estimate curvature. Zr. Ti. O 4 c Zr. O 2 Zr. Ti. O 4 1590° 1610° 1580° Ti. O 2 1610° Al 2 O 3 Al 2 Ti. O 5 Click to continue cc 1590° 1580° Al 2 O 3 c cc

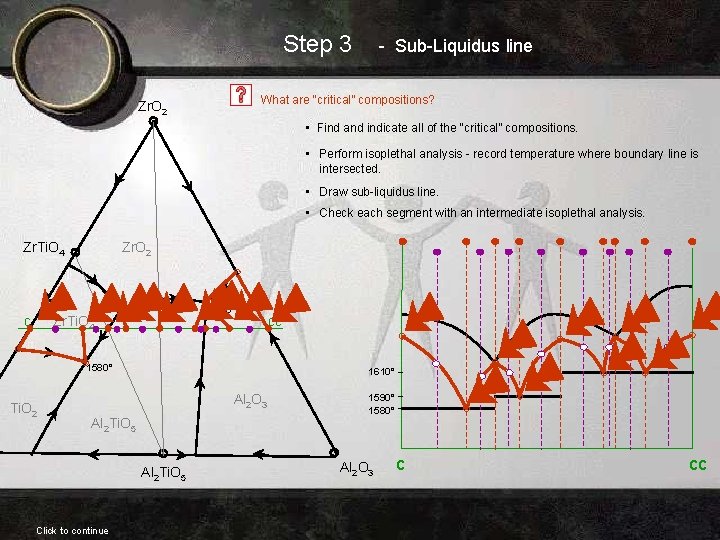
Step 3 - Sub-Liquidus line What are “critical” compositions? Zr. O 2 • Find and indicate all of the “critical” compositions. • Perform isoplethal analysis - record temperature where boundary line is intersected. • Draw sub-liquidus line. • Check each segment with an intermediate isoplethal analysis. Zr. Ti. O 4 c Zr. O 2 Zr. Ti. O 4 1590° 1610° 1580° Ti. O 2 1610° Al 2 O 3 Al 2 Ti. O 5 Click to continue cc 1590° 1580° Al 2 O 3 c cc

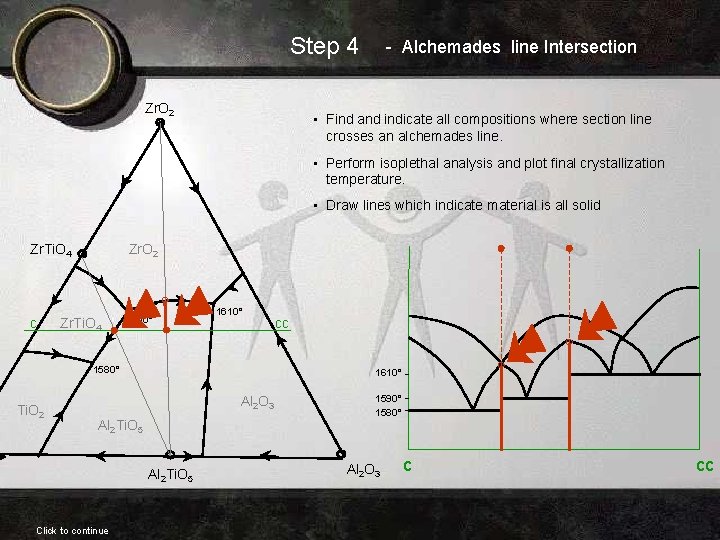
Step 4 Zr. O 2 - Alchemades line Intersection • Find and indicate all compositions where section line crosses an alchemades line. • Perform isoplethal analysis and plot final crystallization temperature. • Draw lines which indicate material is all solid Zr. Ti. O 4 c Zr. O 2 Zr. Ti. O 4 1590° 1610° 1580° Ti. O 2 1610° Al 2 O 3 Al 2 Ti. O 5 Click to continue cc 1590° 1580° Al 2 O 3 c cc

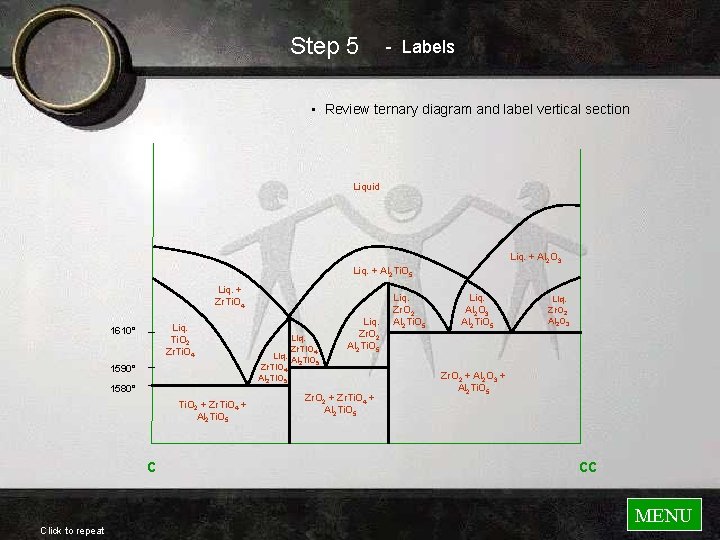
Step 5 - Labels • Review ternary diagram and label vertical section Liquid Liq. + Al 2 O 3 Liq. + Al 2 Ti. O 5 Liq. + Zr. Ti. O 4 Liq. Ti. O 2 Zr. Ti. O 4 1610° 1590° 1580° Ti. O 2 + Zr. Ti. O 4 + Al 2 Ti. O 5 c Click to repeat Liq. Zr. Ti. O 4 Liq. Al Ti. O 2 5 Zr. Ti. O 4 Al 2 Ti. O 5 Liq. Zr. O 2 Al 2 Ti. O 5 Zr. O 2 + Zr. Ti. O 4 + Al 2 Ti. O 5 Liq. Zr. O 2 Al 2 Ti. O 5 Liq. Al 2 O 3 Al 2 Ti. O 5 Liq. Zr. O 2 Al 2 O 3 Zr. O 2 + Al 2 O 3 + Al 2 Ti. O 5 cc MENU