Constant Pressure Heating of TwoPhase Substance P M

Constant Pressure Heating of Two-Phase Substance P M V Subbarao Professor Mechanical Engineering Department I I T Delhi Most Powerful Solution for Many Extrasomatic Needs…. .
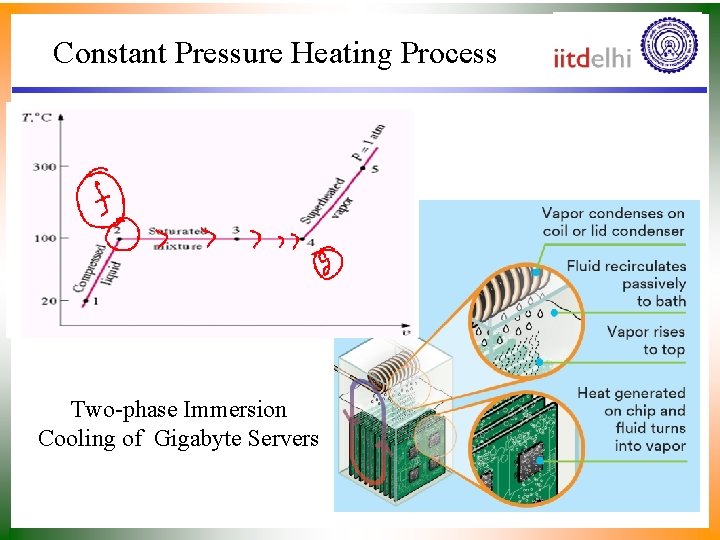
Constant Pressure Heating Process Two-phase Immersion Cooling of Gigabyte Servers
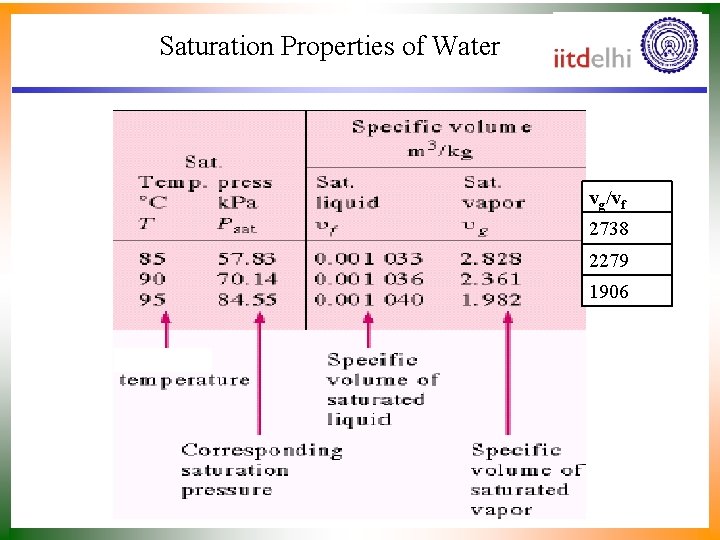
Saturation Properties of Water vg/vf 2738 2279 1906
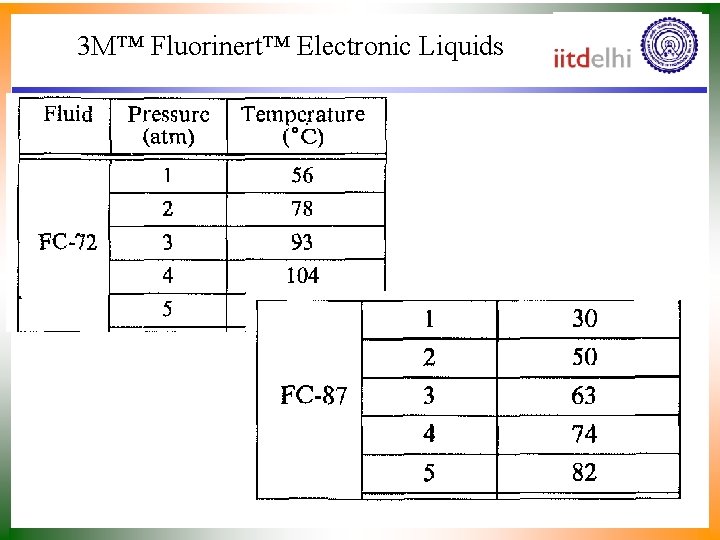
3 M™ Fluorinert™ Electronic Liquids

Wet Vapour • • • Pressure and temperature are not independent properties. Either p & V or T& V are independent pair. P & v or T & v can also be considered. A new property is defined for steam for ease of design. This is called Quality of wet steam.
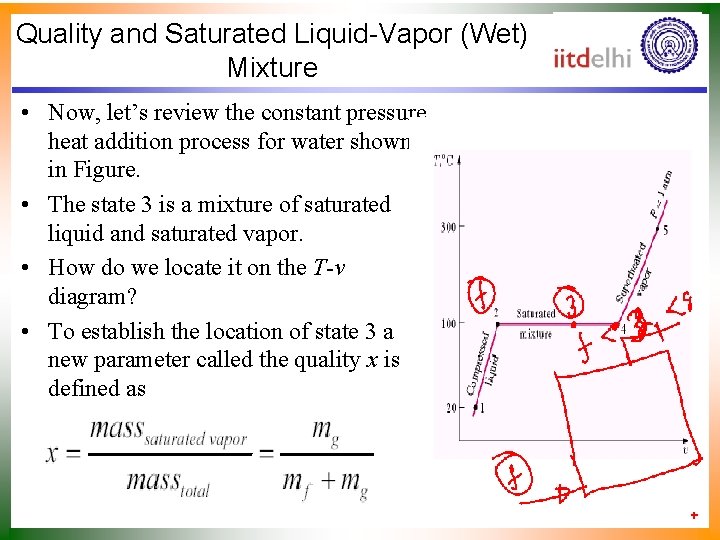
Quality and Saturated Liquid-Vapor (Wet) Mixture • Now, let’s review the constant pressure heat addition process for water shown in Figure. • The state 3 is a mixture of saturated liquid and saturated vapor. • How do we locate it on the T-v diagram? • To establish the location of state 3 a new parameter called the quality x is defined as

Quality and Saturated Liquid-Vapor (Wet) Mixture • The quality is zero for the saturated liquid and one for the saturated vapor (0 x 1). • The average specific volume at any state 3 is given in terms of the quality as follows. • Consider a mixture of saturated liquid and saturated vapor. • The liquid has a mass mf and occupies a volume Vf. • The vapor has a mass mg and occupies a volume Vg.
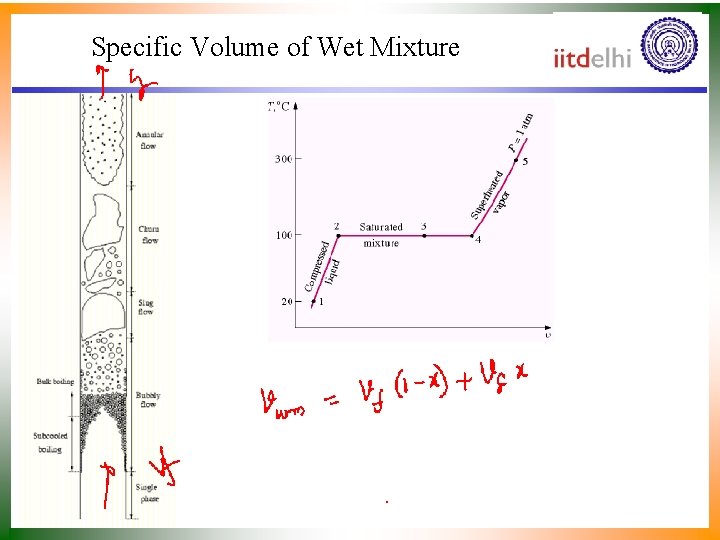
Specific Volume of Wet Mixture
The Theory of Producing Steam/Vapour • Water and steam are typically used as heat carriers in heat treatment systems. • Water boils and evaporates at 100°C under atmospheric pressure. • By higher pressure, water evaporates at higher temperature - e. g. a pressure of 10 bar equals an evaporation temperature of ~179. 90 C. • At a constant pressure of 10 MPa the saturation temperature is 311. 10 C.
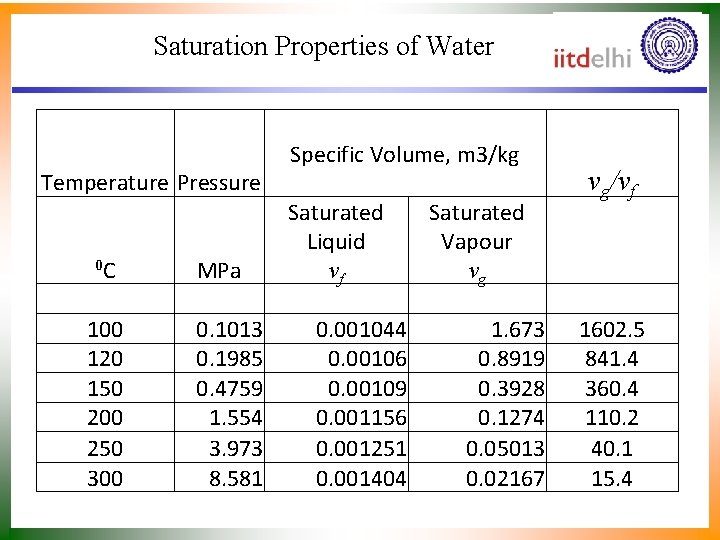
Saturation Properties of Water Temperature Pressure 0 C 100 120 150 200 250 300 MPa 0. 1013 0. 1985 0. 4759 1. 554 3. 973 8. 581 Specific Volume, m 3/kg Saturated Liquid vf 0. 001044 0. 00106 0. 00109 0. 001156 0. 001251 0. 001404 Saturated Vapour vg 1. 673 0. 8919 0. 3928 0. 1274 0. 05013 0. 02167 vg/vf 1602. 5 841. 4 360. 4 110. 2 40. 1 15. 4
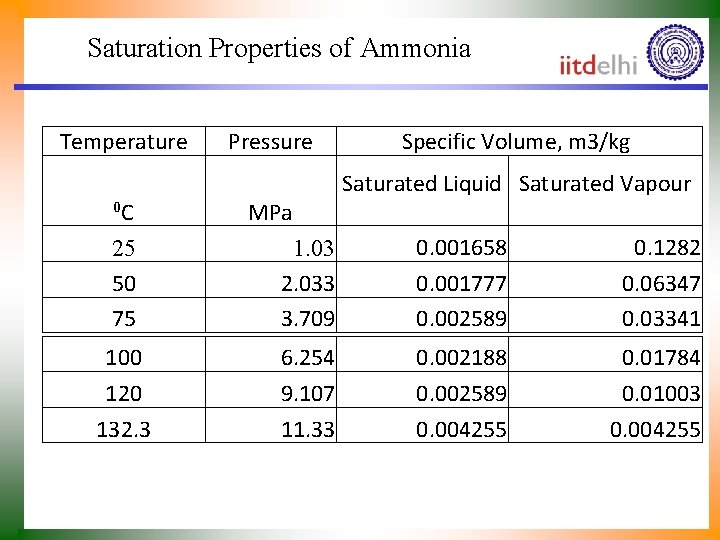
Saturation Properties of Ammonia Temperature 0 C Pressure MPa Specific Volume, m 3/kg Saturated Liquid Saturated Vapour 25 50 75 1. 03 2. 033 3. 709 0. 001658 0. 001777 0. 002589 0. 1282 0. 06347 0. 03341 100 120 132. 3 6. 254 9. 107 11. 33 0. 002188 0. 002589 0. 004255 0. 01784 0. 01003 0. 004255

Superposition of Many Constant Pressure Processes • If all of the saturated liquid states are connected, the saturated liquid line is established. • If all of the saturated vapor states are connected, the saturated vapor line is established. • These two lines intersect at the critical point and form what is often called the “steam dome. ” The critical point of water is 374. 14 o. C, 22. 09 MPa The critical point of ammonia is 132. 3 o. C, 11. 33 MPa
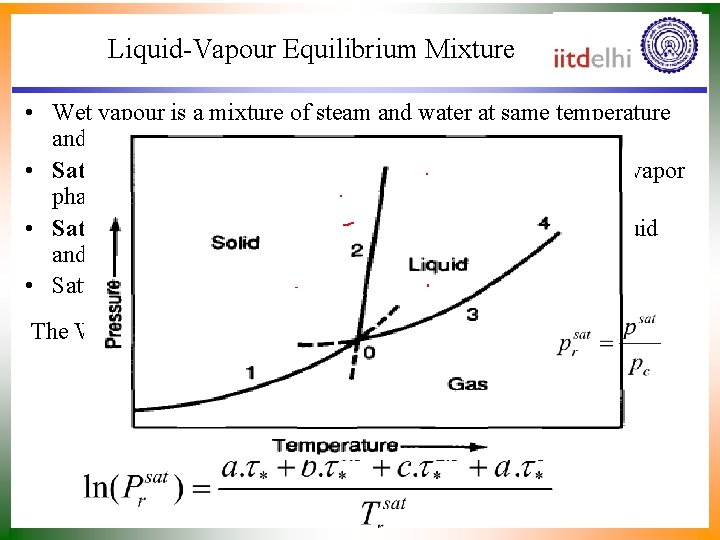
Liquid-Vapour Equilibrium Mixture • Wet vapour is a mixture of steam and water at same temperature and pressure. • Saturation pressure is the pressure at which the liquid and vapor phases are in equilibrium at a given temperature. • Saturation temperature is the temperature at which the liquid and vapor phases are in equilibrium at a given pressure. • Saturation Pressure is function of temperature or vice versa. T = F(p) The Wagner-Ambrose equation
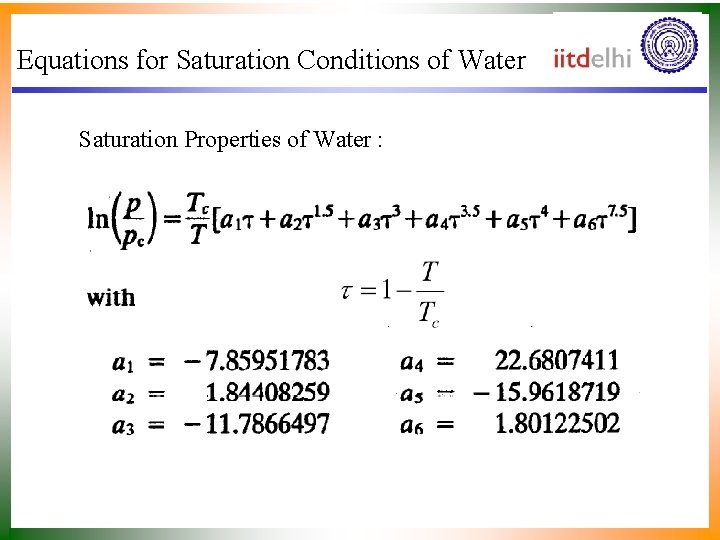
Equations for Saturation Conditions of Water Saturation Properties of Water :
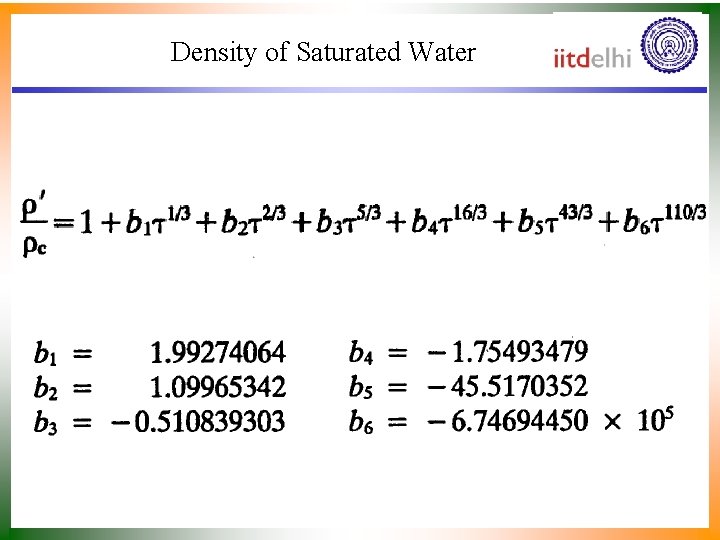
Density of Saturated Water
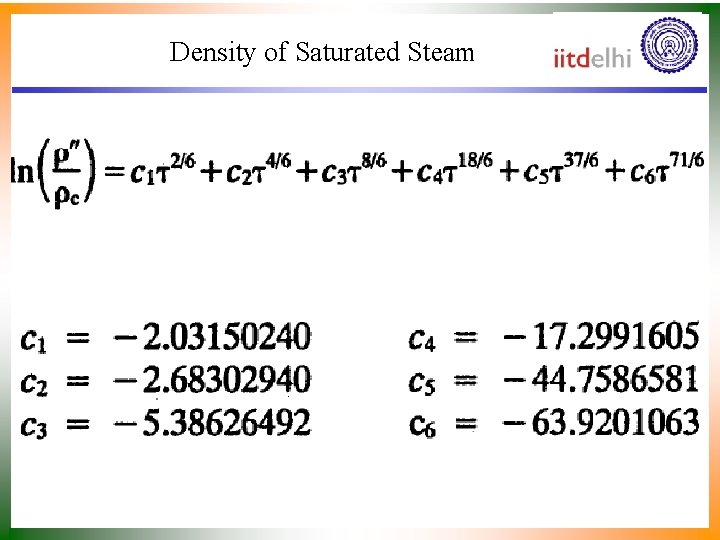
Density of Saturated Steam
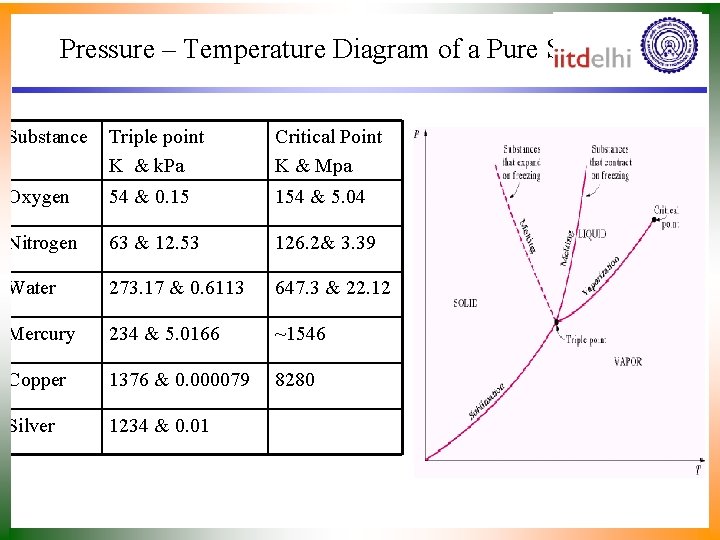
Pressure – Temperature Diagram of a Pure Substance Triple point K & k. Pa Critical Point K & Mpa Oxygen 54 & 0. 15 154 & 5. 04 Nitrogen 63 & 12. 53 126. 2& 3. 39 Water 273. 17 & 0. 6113 647. 3 & 22. 12 Mercury 234 & 5. 0166 ~1546 Copper 1376 & 0. 000079 8280 Silver 1234 & 0. 01
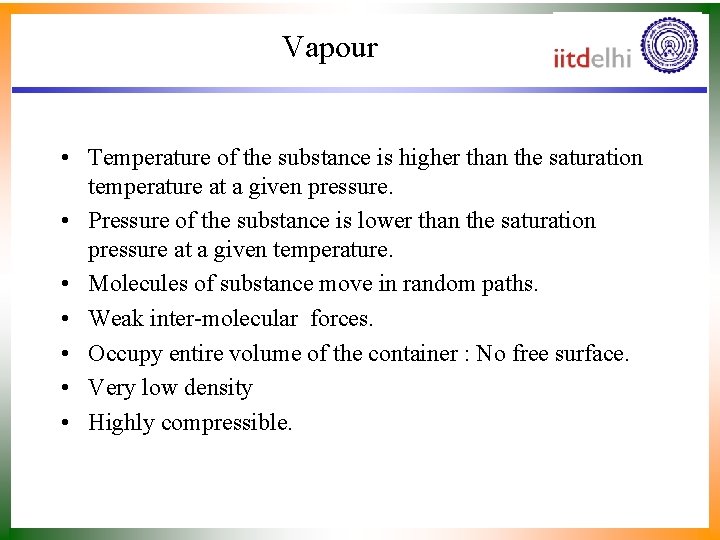
Vapour • Temperature of the substance is higher than the saturation temperature at a given pressure. • Pressure of the substance is lower than the saturation pressure at a given temperature. • Molecules of substance move in random paths. • Weak inter-molecular forces. • Occupy entire volume of the container : No free surface. • Very low density • Highly compressible.
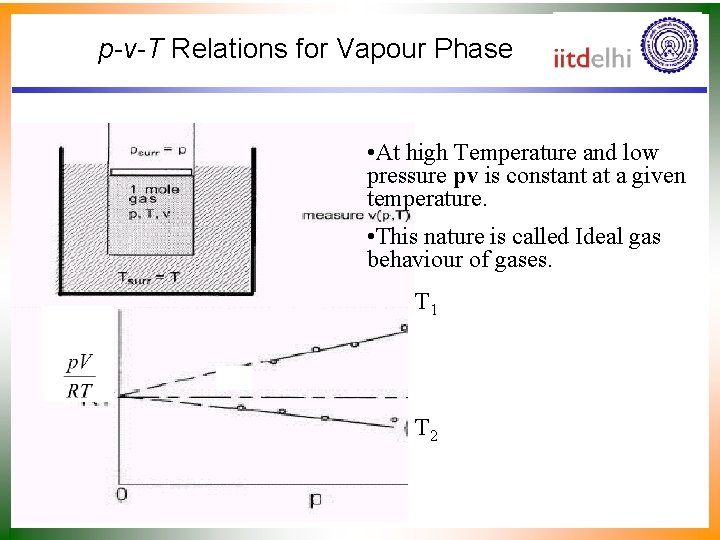
p-v-T Relations for Vapour Phase • At high Temperature and low pressure pv is constant at a given temperature. • This nature is called Ideal gas behaviour of gases. T 1 T 2
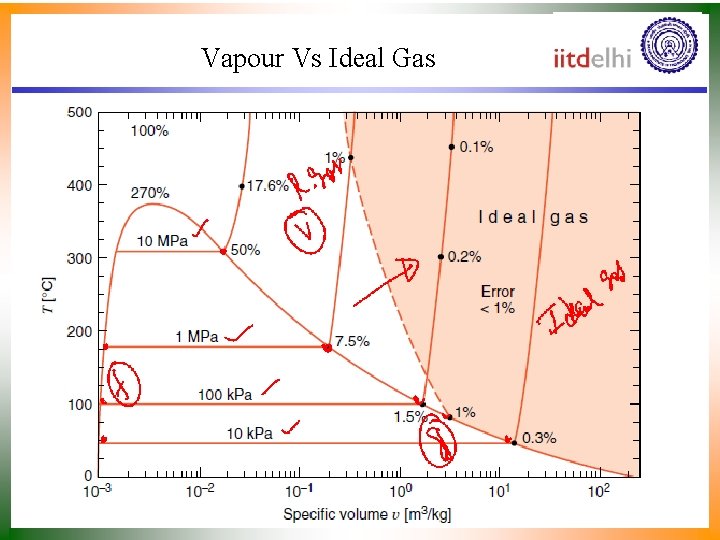
Vapour Vs Ideal Gas
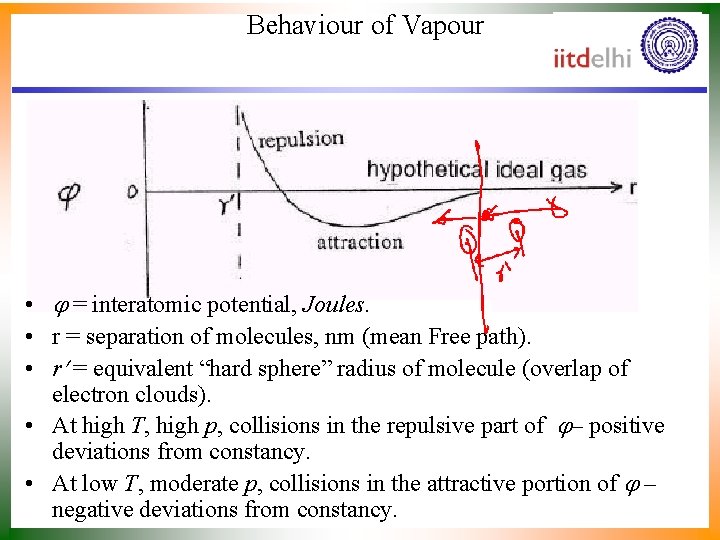
Behaviour of Vapour • = interatomic potential, Joules. • r = separation of molecules, nm (mean Free path). • r = equivalent “hard sphere” radius of molecule (overlap of electron clouds). • At high T, high p, collisions in the repulsive part of – positive deviations from constancy. • At low T, moderate p, collisions in the attractive portion of – negative deviations from constancy.
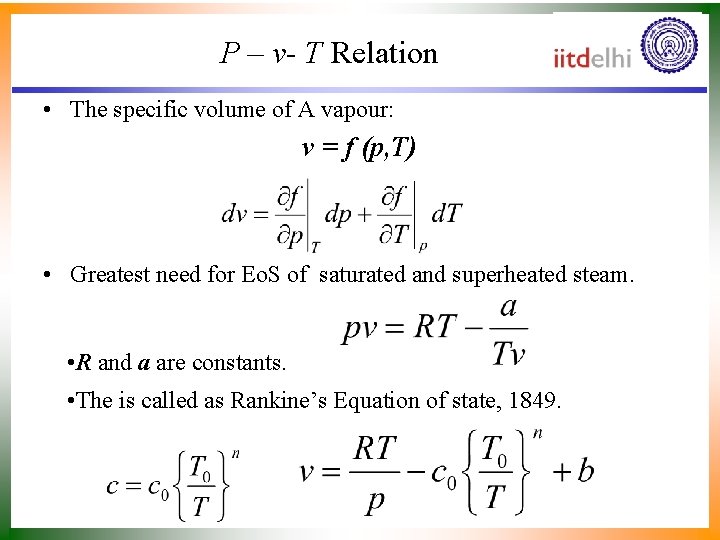
P – v- T Relation • The specific volume of A vapour: v = f (p, T) • Greatest need for Eo. S of saturated and superheated steam. • R and a are constants. • The is called as Rankine’s Equation of state, 1849.
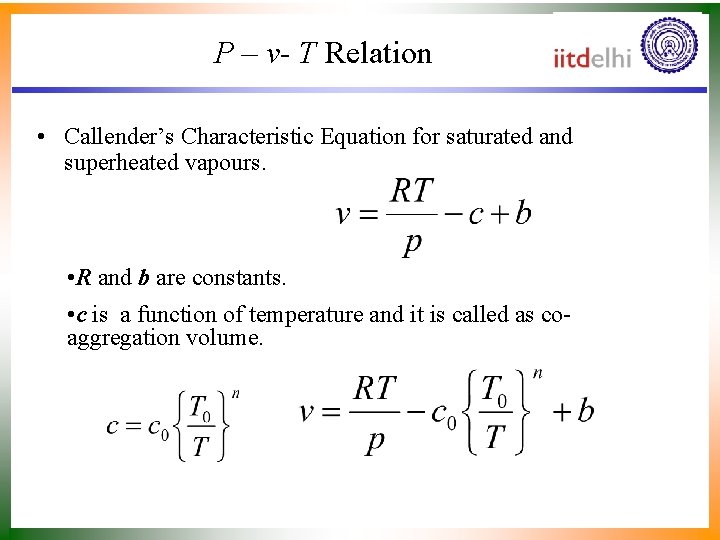
P – v- T Relation • Callender’s Characteristic Equation for saturated and superheated vapours. • R and b are constants. • c is a function of temperature and it is called as coaggregation volume.
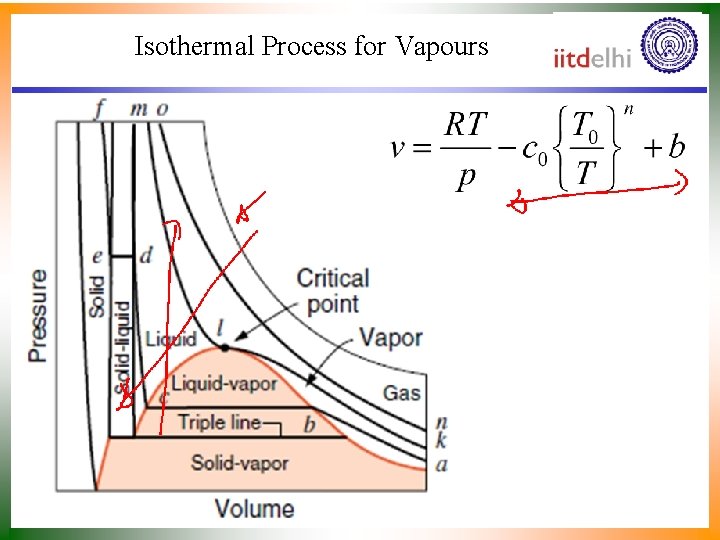
Isothermal Process for Vapours
JO H A N N E S D. V A N D E R W A A L S The equation of state for gases and liquids Nobel Lecture, December 12, 1910 I intend to discuss in sequence: (1) the broad outlines of my equation of state and how I arrived at it; (2) what my attitude was and still is to that equation; (3) how in the last four years I have sought to account for the discrepancies which remained between the experimental results and this equation; (4) how I have also sought to explain the behaviour of binary and ternary mixtures by means of the equation of state.
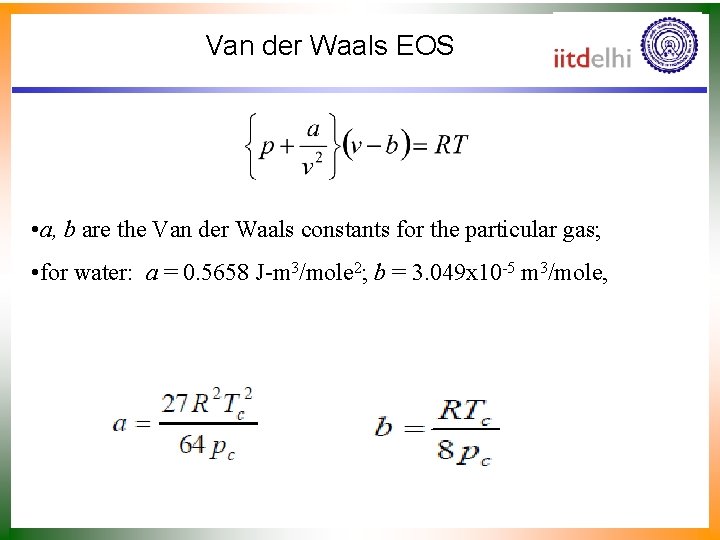
Van der Waals EOS • a, b are the Van der Waals constants for the particular gas; • for water: a = 0. 5658 J-m 3/mole 2; b = 3. 049 x 10 -5 m 3/mole,
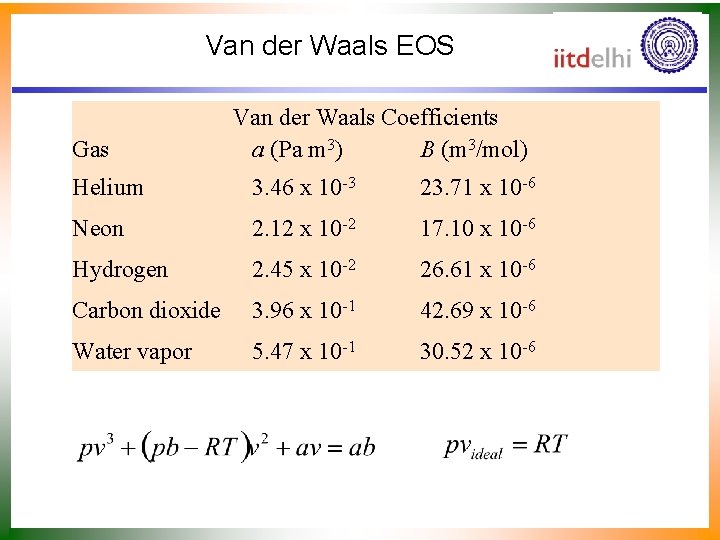
Van der Waals EOS Gas Van der Waals Coefficients a (Pa m 3) B (m 3/mol) Helium 3. 46 x 10 -3 23. 71 x 10 -6 Neon 2. 12 x 10 -2 17. 10 x 10 -6 Hydrogen 2. 45 x 10 -2 26. 61 x 10 -6 Carbon dioxide 3. 96 x 10 -1 42. 69 x 10 -6 Water vapor 5. 47 x 10 -1 30. 52 x 10 -6
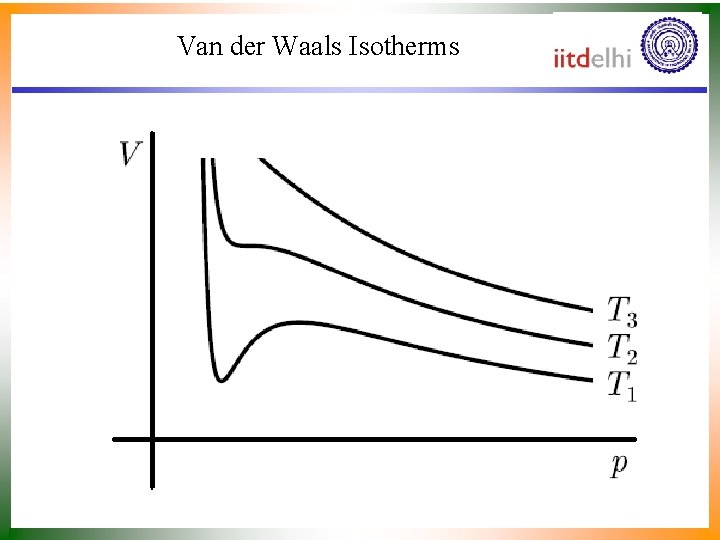
Van der Waals Isotherms
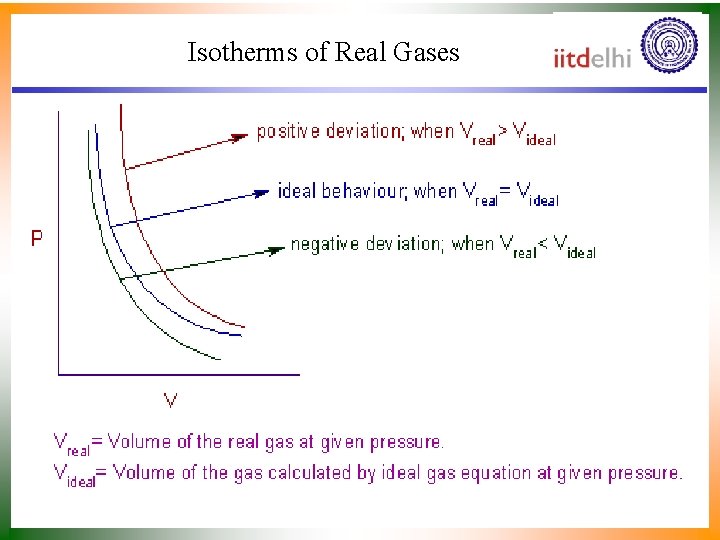
Isotherms of Real Gases
- Slides: 29