CONFLICT OF INTEREST TRAINING AHCCCS Pharmacy and Therapeutics
















- Slides: 18

CONFLICT OF INTEREST TRAINING AHCCCS Pharmacy and Therapeutics Committee · October 2019

AHCCCS Statewide Drug List Pharmacy and Therapeutics Advisory Committee Ethical Duties and Constraints Three-Part Interrelationship

AHCCCS Drug List A list of preferred drugs to be used by all Contractors responsible for the administration of acute and longterm care pharmacy benefits. This drug list identifies specific federally and state reimbursable medications and related products, which are supported by current evidence-based medicine. The AHCCCS Drug List was developed to encourage the use of safe, effective, clinically appropriate, and the most costeffective medications.

PHARMACY & THERAPEUTICS ADVISORY COMMITTEE Duties and Responsibilities: - Make recommendations to AHCCCS on developing, managing, updating, and administering the AHCCCS Drug List and prior authorization criteria. - Evaluate scientific evidence of the relative safety, efficacy, effectiveness, and clinical appropriateness of prescription drugs.

ETHICAL DUTIES AND CONSTRAINTS Government ethics address the inevitable conflict between public good and personal gain. Conflicts of interest present themselves in virtually all government endeavors. The purpose of ethical rules is to prevent and resolve conflicts of interest.

WHAT ARE THE P&T COMMITTEE’S ETHICAL RULES DESIGNED TO PROTECT? It’s all in the adjectives: Medications and related products supported by current evidence-based medicine. The AHCCCS Drug List was developed to encourage the use of safe, effective, clinically appropriate, and the most cost-effective medications.

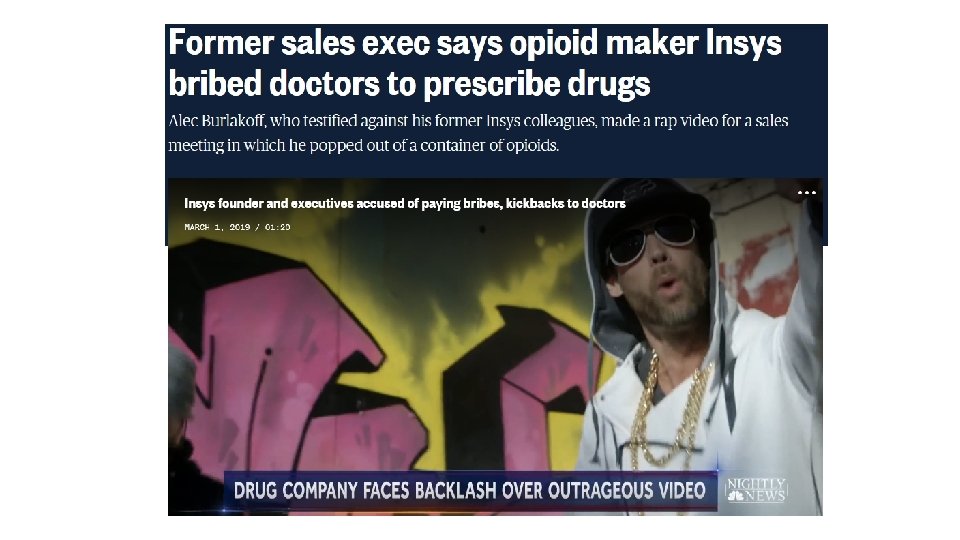
HOW CONFLICTS ARISE Manufacturers use sales representatives and marketing teams. They have the information you need to evaluate whether evidence supports use of a drug for its indication; whether it’s safe, effective, and so forth. Their purpose is to sell. AHCCCS, per your advice, permits providers to prescribe their products. Your purpose is to evaluate. Potential Conflict: Incentives to approve drugs that do not meet the criteria.

ARE ETHICAL RULES NECESSARY IN THIS CONTEXT? How do manufacturers increase sales and market share? How do they train their sales teams to market their drugs? How do they convince providers to use their drugs? How might they convince Committee members to list their drugs?



THE P&T COMMITTEE AS AN INTERMEDIARY We do need pharmaceutical companies to bring drugs to market We do expect pharmaceutical companies to promote their products. The cost to develop one new drug is $2. 6 billion; approval rate is <12% Source: https: //www. policymed. com/2014/12/a-tough-road-cost-to-develop-one-new-drug-is-26 -billion-approval-rate-for-drugs-entering-clinical -de. html We do expect drug manufacturers to make truthful representations. We do rely on drug marketers for information. We do not expect objectivity from drug manufacturers.

AHCCCS Contractor Operations Manual Ch. 111 Section III(B)(4) Committee members shall not: a. Be employed by, subcontract with, or directly or indirectly represent a pharmaceutical manufacturer, b. Be employed by, subcontract with, or directly or indirectly represent a Pharmacy Benefits Management (PBM) company, or c. Receive payments or compensation from the pharmaceutical industry in excess of the physician mean general payment amount for the most recent year as specified on openpaymentsdata. cms. gov.

CLOSER QUESTIONS P & T Committee members cannot be employed by, subcontract with, or directly or indirectly represent a pharmaceutical manufacturer or a PBM What are subcontracts? Consulting? Honoraria? Licensing/royalty fees? CMS treats all these as types of payments for calculating the mean If you are speak at a continuing education program about a drug manufactured by the sponsor, do you directly or indirectly represent the manufacturer? What if your spouse is employed by, or subcontracts with a manufacturer?

EXECUTIVE ORDER 201806: Five Provisions 1. ) The Arizona Health Care Cost Containment System shall annually provide mandatory conflict of interest training to all members of the Pharmacy and Therapeutics Committee. 2. ) The Arizona Health Care Cost Containment System shall publicly post all disclosure forms for all members of the Pharmacy and Therapeutics Committee must abstain from vote if conflict of interest exists. 3. ) In accordance with Arizona Revised Statutes Title 38 Chapter 3 Article 8, any member of the Pharmacy and Therapeutics Committee who has a conflict on any agenda item shall abstain from participating in the discussion of and voting on that agenda item. 4. ) The Arizona Health Care Cost Containment System shall adopt a policy requiring any individual providing in-person or written testimony at meetings of the Pharmacy and Therapeutics Committee to disclose whether they represent a pharmaceutical or medical device company and whether they have received any form of payment or compensation from a pharmaceutical or medical device company. 5. ) The Arizona Health Care Cost Containment System shall review publicly available data on payments by pharmaceutical and medical device companies to physicians to determine an appropriate threshold above which a person shall be disqualified from serving on the Pharmacy and Therapeutics Committee.

WHEN MUST YOU ABSTAIN? P & T Committee members with “a conflict on any agenda item” must abstain Example 1: You own stock in Pfizer, and the Committee is discussing whether to delete Prevnar from the list due to reported correlations between vaccinations and Guillan-Barre Syndrome. Can you participate in the discussion? Does it matter how much Pfizer stock you own? BTW, is it on your disclosure form? What if the same public concern applies equally to all vaccines? Example 2: You’re a physician who prefers Prevnar to Pneumovax, or vice versa. Example 3: You’re a paid consultant on Prevnar (or Pneumovax). Example 4: Your nephew’s wife is a Merck employee.

TESTIMONIAL DISCLOSURES TO THE COMMITTEE Persons testifying at P & T Committee meetings must disclose whether they represent or are compensated by a pharmaceutical or medical device company ♦ Not a disqualifier ♦ What percentage of testimony before the P & T Committee is not from marketers? - Legal analog: Testimonial challenges go to admissibility or weight of evidence ♦ Skepticism versus cynicism ♦ Vigorous examination

A. R. S. § 38 -501 et seq. Government Ethics Laws that Apply to All Public Officers A. R. S. § 38 -503 requires disclosure and abstention as set forth in Executive Order 2018 -06. It pertains to a “substantial interest in” a contract, sale, purchase or service to the public agency. A. R. S. § 38 -504(A) You can’t represent, for compensation, any person or entity to the Committee while serving or for one year afterward. A. R. S. § 38 -504(B) You can’t disclose or use, for profit, confidential information obtained while on the committee or for two years afterward. A. R. S. § 38 -504(C) You can’t use your Committee member status to “secure any valuable thing or valuable benefit” if it “is of such character as to manifest a substantial and improper influence on” your duties as a committee member.

QUESTIONS OR ADDITIONAL DISCUSSION