Conductometry Conductometric Analysis Fundamentals of conductometry Conductivity measurements






- Slides: 18

Conductometry (电导法)

Conductometric Analysis • Fundamentals of conductometry • Conductivity measurements • Analytical applications of conductometric measurements

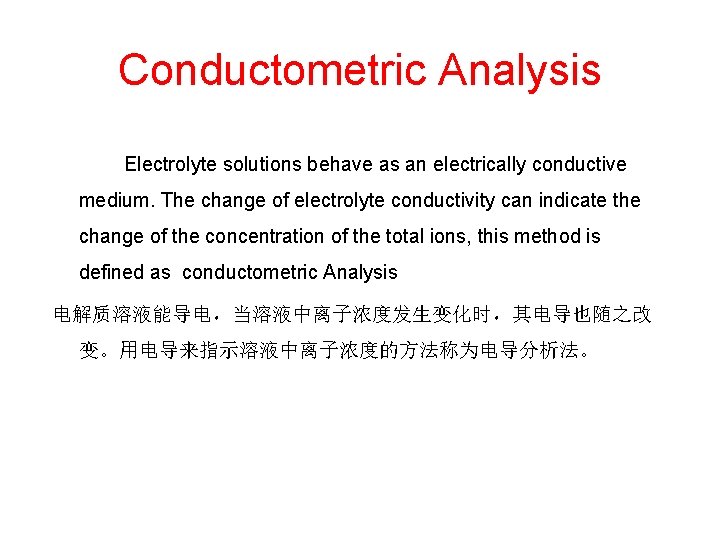
Conductometric Analysis Electrolyte solutions behave as an electrically conductive medium. The change of electrolyte conductivity can indicate the change of the concentration of the total ions, this method is defined as conductometric Analysis 电解质溶液能导电,当溶液中离子浓度发生变化时,其电导也随之改 变。用电导来指示溶液中离子浓度的方法称为电导分析法。

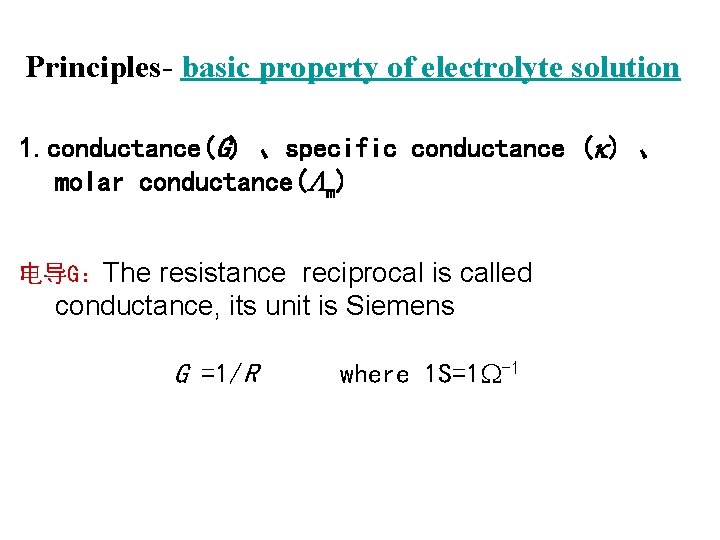
Principles- basic property of electrolyte solution 1. conductance(G) 、specific conductance ( ) 、 molar conductance( m) 电导G:The resistance reciprocal is called conductance, its unit is Siemens G =1/R where 1 S=1 -1

电导率 : The resistivity reciprocal is specific conductance or electrolytic conductivity = G L / A its unit :S cm-1 两电极板为单位面积,距离为单位长度时溶液的 电导。

Ratio L/A is an intrinsic parameter of each conductometric container and is called resistive capacity of conductometric container θ θ=L/A 电导池常数θ:θ = L / A (A the surface area of electrodes; L the distance between electrodes) 由标准KCl溶液的电导率(查表)确定电导率和电导池常 数

摩尔电导率( m) Because specific conductance depends on concentration we introduce the term molar conductance m Units:S cm 2 mol-1 距离为单位长度的两电极板间含有单位物质的量的电解质的 溶液的电导。 不同浓度、不同类型电解质导电能力的比较。

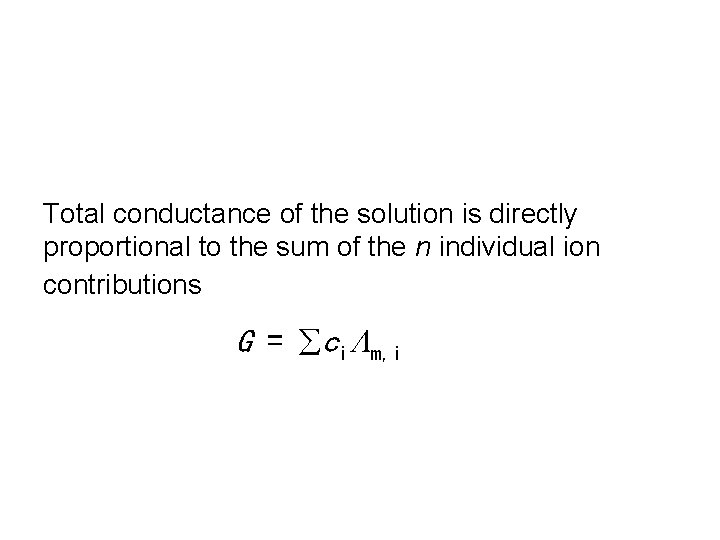
Total conductance of the solution is directly proportional to the sum of the n individual ion contributions G = ci m, i

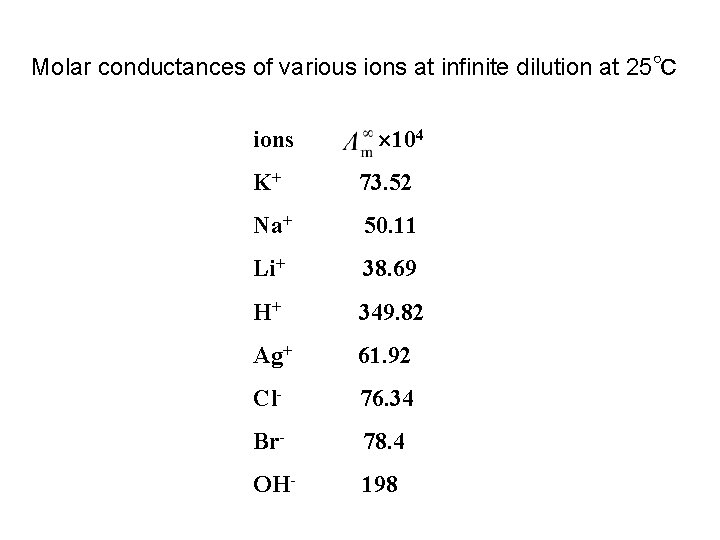
2. Conductance of ions离子的电导 无限稀释摩尔电导率 Molar conductance at infinite dilution is the characteristic constant for given electrolyte

Molar conductances of various ions at infinite dilution at 25℃ ions 104 K+ 73. 52 Na+ 50. 11 Li+ 38. 69 H+ 349. 82 Ag+ 61. 92 Cl- 76. 34 Br- 78. 4 OH- 198


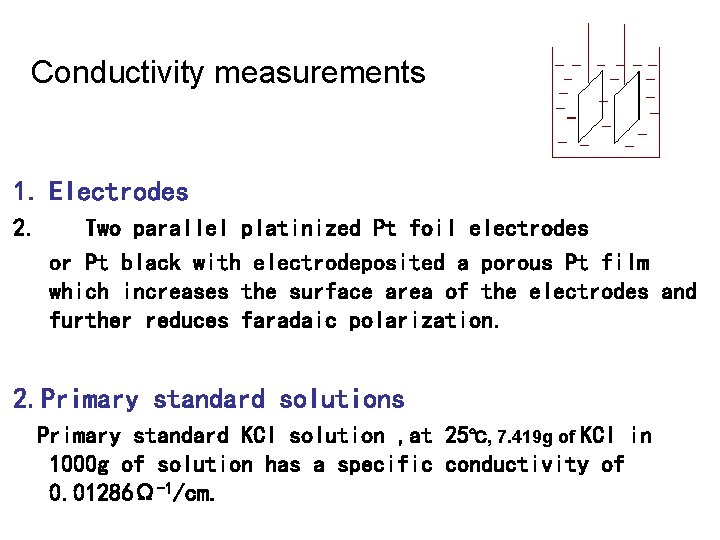
Conductivity measurements 1. Electrodes 2. Two parallel platinized Pt foil electrodes or Pt black with electrodeposited a porous Pt film which increases the surface area of the electrodes and further reduces faradaic polarization. 2. Primary standard solutions Primary standard KCl solution , at 25℃, 7. 419 g of KCl in 1000 g of solution has a specific conductivity of 0. 01286Ω-1/cm.

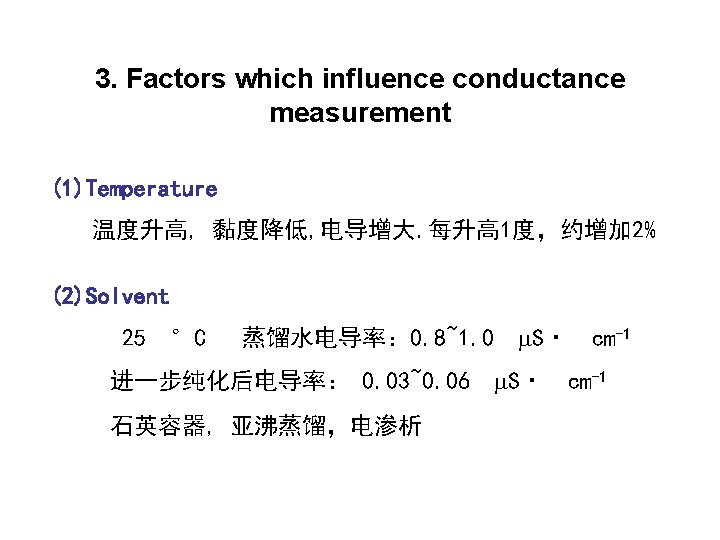
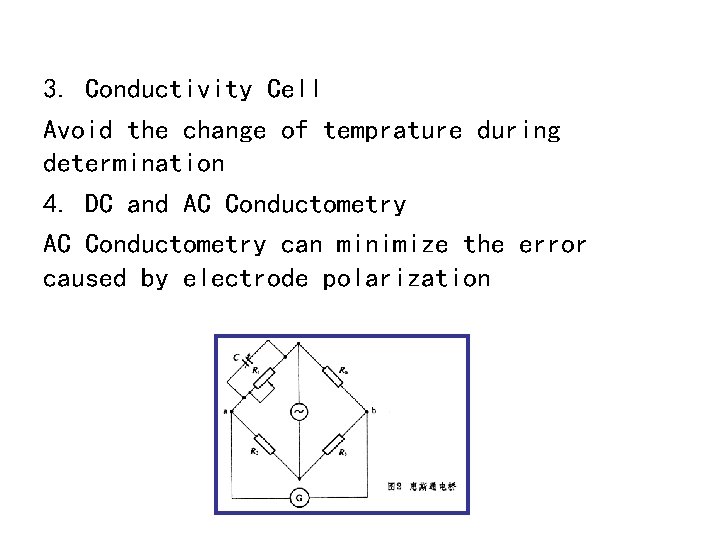
3. Conductivity Cell Avoid the change of temprature during determination 4. DC and AC Conductometry can minimize the error caused by electrode polarization

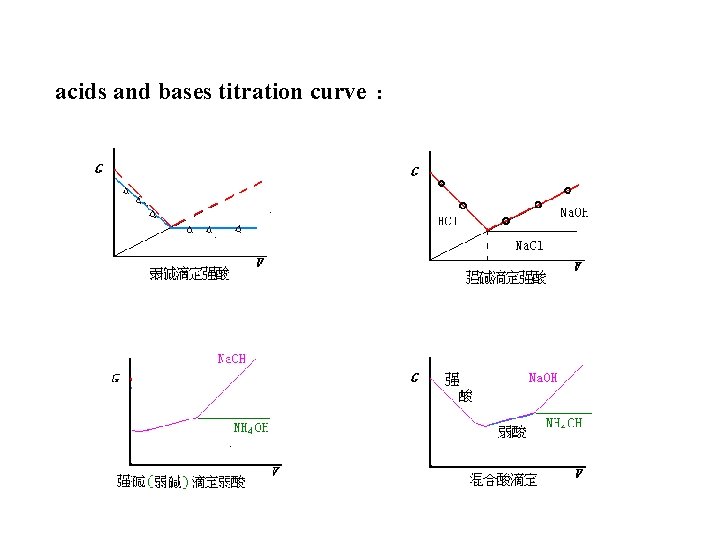
Applications of Conductometric Analysis 1. conductometric titration电导滴定 conductometric titration is usually used in the determination of dilute acids, weak acids and mixed acids 电导滴定常用于稀酸、弱酸、混合酸等的测定。

acids and bases titration curve :

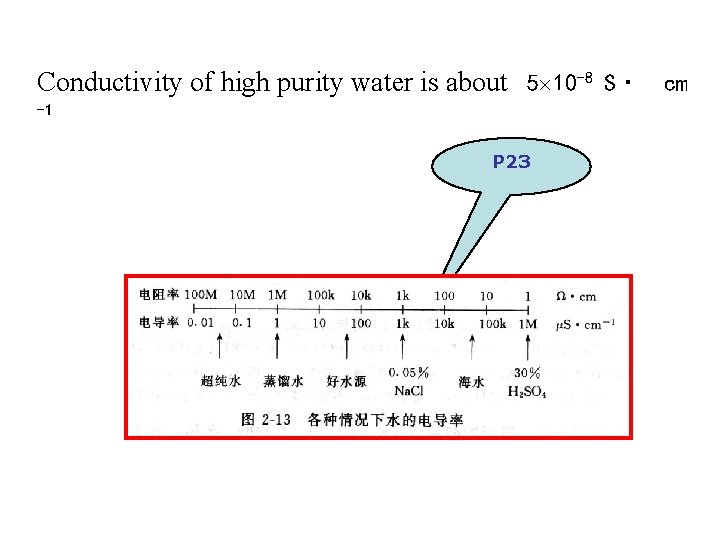
2. application of direct conductometry直接电导法应用 G = Kc Calibration curves and standard-addition methods a. Determination of high purity water Conductivity is used to determine the purity of drink water , natural water, deionized water and wastewater

Conductivity of high purity water is about 5 10 -8 S· cm -1 P 23

• b. Determination of the total concentration of strong electrolyte solutions salinity of soil and sea water • c. Determination of gaseous environmental contaminant in air eg. SO 3 NO 2, acidic rain determine the change of conductivity after absorption