CONCLUSION to the BLUE LAB How do we

CONCLUSION to the BLUE LAB!

How do we know chemical reaction happened yesterday? 1. 2. 3. 4.

How do we know chemical reaction happened yesterday? 1. major, unexplained, color change 2. major, unexplained temperature change 3. major, unexplained bubbles gas created 4. precipitate forms.

• Go over rest of sheet…

What are some types of chemical reactions?

We have already learned about 3 general types of chemical reactions: 1. A. B. 2. 3.

Which two below need oxygen? 1. OXIDATION reactions NEED O 2 as REACTANT A. B. 2. 3.

Which two below need oxygen? 1. OXIDATION reactions NEED O 2 as REACTANT A. combustion B. corrosion 2. 3.

What are the two ways heat &/or other energy can flow in & out of a reaction? 1. OXIDATION reactions NEED O 2 as REACTANT A. combustion/fire B. corrosion/rusting 2. E 3. E

We have already learned about 3 general types of chemical reactions: 1. OXIDATION reactions NEED O 2 as REACTANT A. combustion/fire B. corrosion/rusting 2. EXOTHERMIC reactions RELEASE HEAT (or other energy)! 3. ENDOTHERMIC reactions ABSORB HEAT!

Can you recognize which type of reaction it is just by its chemical reaction formula? HEAT Ba(OH)2 + 8 H 2 O + 2 NH 4 Cl → 2 NH 3 + 10 H 2 O +Ba. Cl 2

ENDOTHERMIC! This is the chemcial reaction for that cold pack. HEAT Ba(OH)2 + 8 H 2 O + 2 NH 4 Cl → 2 NH 3 + 10 H 2 O +Ba. Cl 2

ENDOTHERMIC! This is the chemcial reaction for that cold pack. HEAT + Ba(OH)2 + 8 H 2 O + 2 NH 4 Cl → 2 NH 3 + 10 H 2 O +Ba. Cl 2

General types of chemical reactions: 1. OXIDATION reactions NEED O 2 as REACTANT A. combustion/fire B. corrosion/rusting 2. EXOTHERMIC reactions RELEASE HEAT (&/or other energy)! 3. ENDOTHERMIC reactions ABSORB HEAT (&/or other energy)!

CHEMICAL REACTIONS CAN FIT MORE THAN ONE CATEGORY…
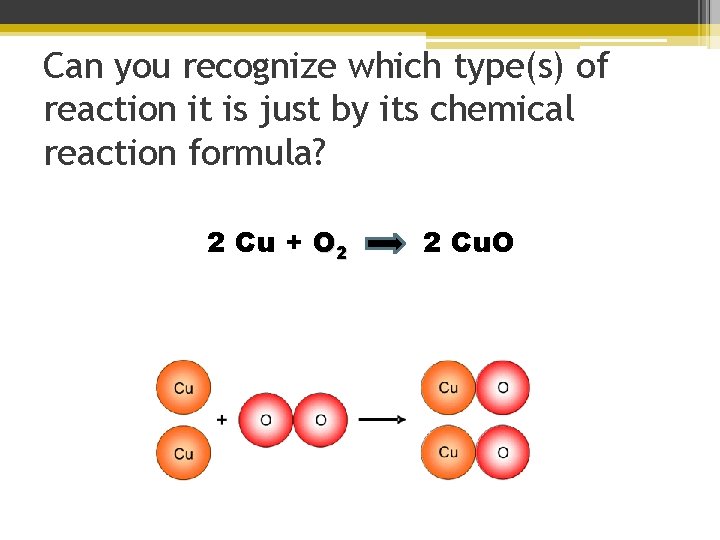
Can you recognize which type(s) of reaction it is just by its chemical reaction formula? 2 Cu + O 2 2 Cu. O
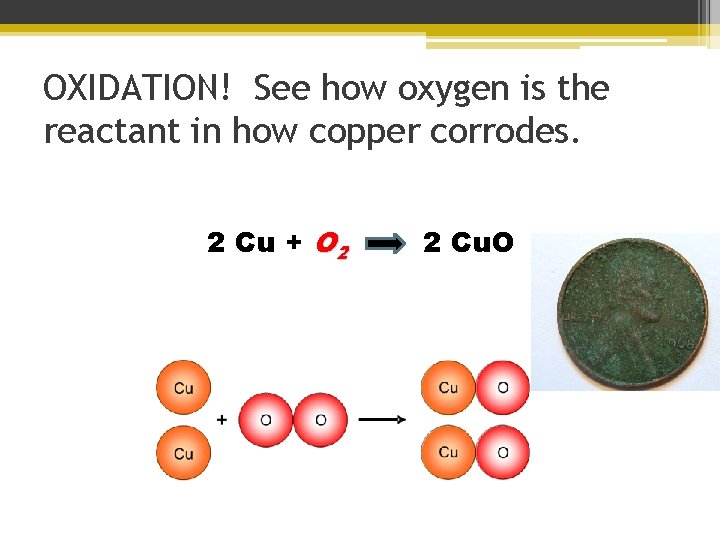
OXIDATION! See how oxygen is the reactant in how copper corrodes. 2 Cu + O 2 2 Cu. O
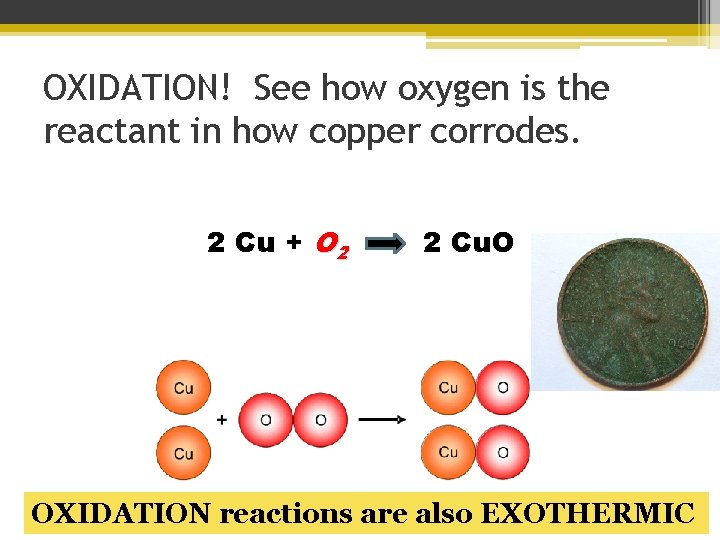
OXIDATION! See how oxygen is the reactant in how copper corrodes. 2 Cu + O 2 2 Cu. O OXIDATION reactions are also EXOTHERMIC

Chemical reactions can fit more than one category… 1. OXIDATION reactions NEED O 2 as reactant A. combustion/fire B. corrosion/rusting/tarnishing 2. EXOTHERMIC reactions RELEASE HEAT! 3. ENDOTHERMIC reactions ABSORB HEAT!

Can you recognize which type of reaction it is just by its chemical reaction formula?
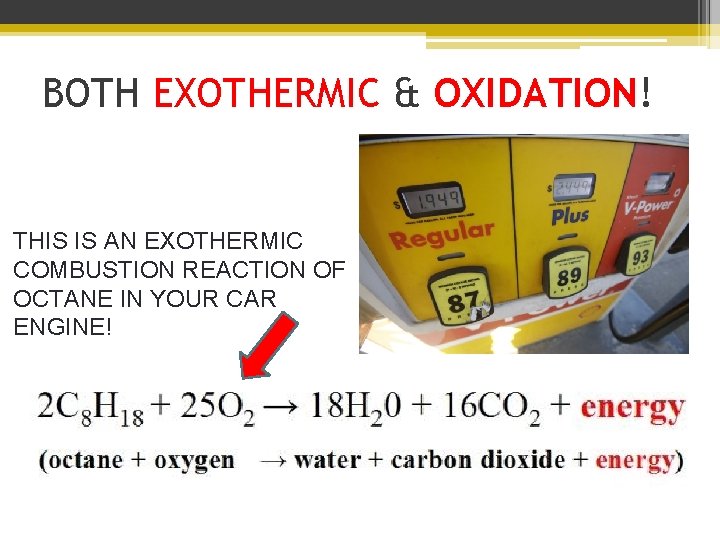
BOTH EXOTHERMIC & OXIDATION! THIS IS AN EXOTHERMIC COMBUSTION REACTION OF OCTANE IN YOUR CAR ENGINE!

ALL OXIDATION reactions are all EXOTHERMIC! 1. OXIDATION reactions NEED O 2 as reactant A. combustion/fire B. corrosion/rusting/tarnishing 2. EXOTHERMIC reactions RELEASE HEAT! 3. ENDOTHERMIC reactions ABSORB HEAT!

YOU COMBUST…. YOU RUST…. YOU ARE EXOTHERMIC!
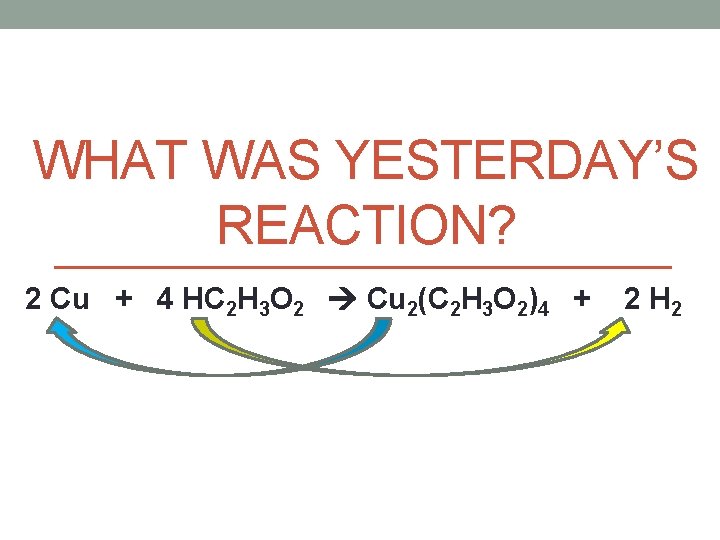
WHAT WAS YESTERDAY’S REACTION? 2 Cu + 4 HC 2 H 3 O 2 Cu 2(C 2 H 3 O 2)4 + 2 H 2

It was EXOTHERMIC for SURE, but not with OXYGEN GAS as a reactant 1. OXIDATION reactions NEED O 2 as reactant A. combustion/fire B. corrosion/rusting/tarnishing 2. EXOTHERMIC reactions RELEASE HEAT! 3. ENDOTHERMIC reactions ABSORB HEAT!
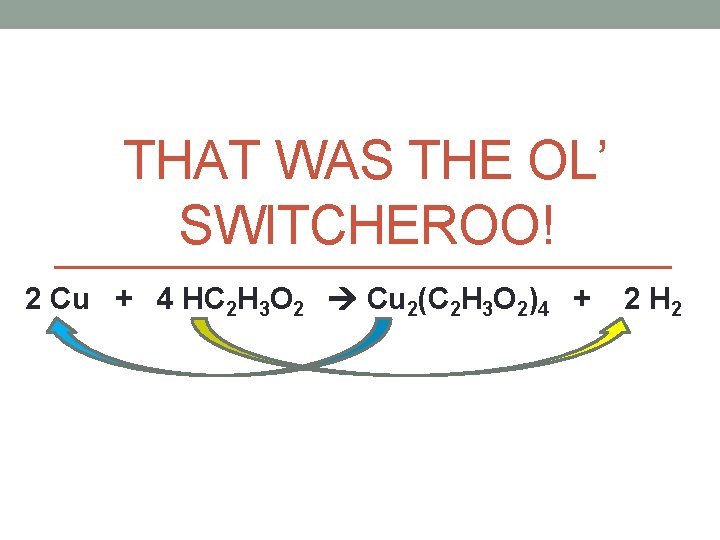
THAT WAS THE OL’ SWITCHEROO! 2 Cu + 4 HC 2 H 3 O 2 Cu 2(C 2 H 3 O 2)4 + 2 H 2

THERE’S A FEW MORE GENERAL TYPES OF CHEMICAL REACTIONS we should know. 1. OXIDATION reactions NEED O 2 as reactant A. combustion B. corrosion 2. EXOTHERMIC reactions RELEASE HEAT! 3. ENDOTHERMIC reactions ABSORB HEAT! 4. REPLACMENT reactions 5. 6.

THERE’S A COUPLE MORE GENERAL TYPES OF CHEMICAL REACTIONS we should know. 1. OXIDATION reactions NEED O 2 as reactant A. combustion B. corrosion 2. EXOTHERMIC reactions RELEASE HEAT! 3. ENDOTHERMIC reactions ABSORB HEAT! 4. REPLACEMENT reactions-switcheroo!! 5. 6.
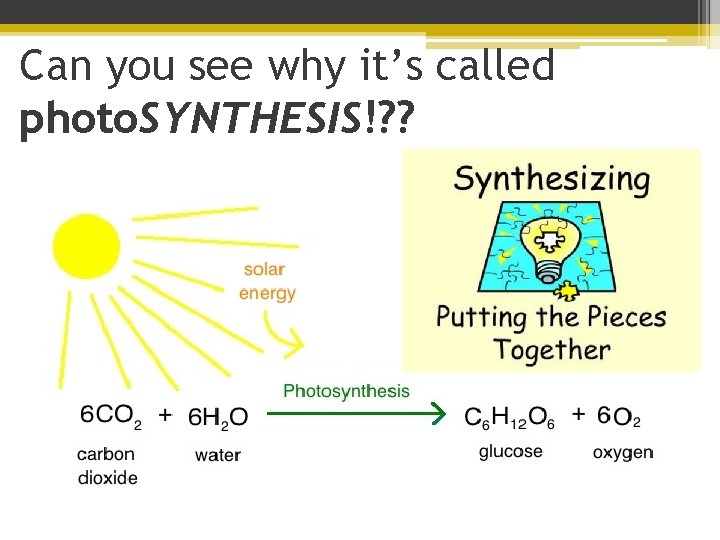
Can you see why it’s called photo. SYNTHESIS!? ?
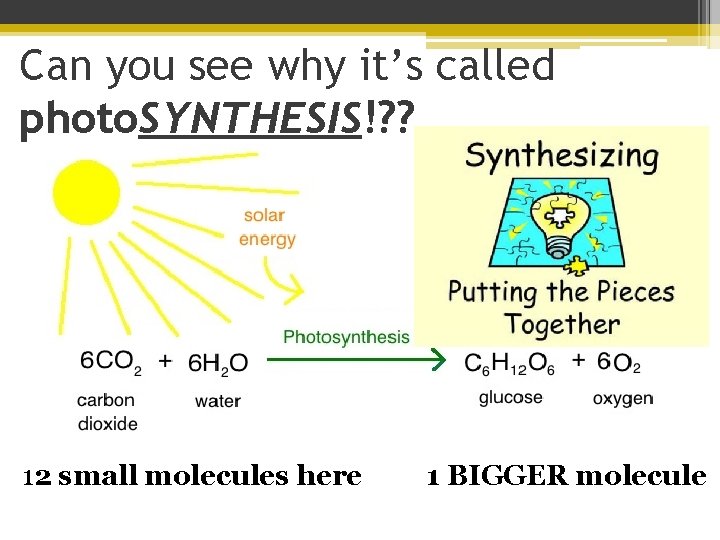
Can you see why it’s called photo. SYNTHESIS!? ? 12 small molecules here 1 BIGGER molecule
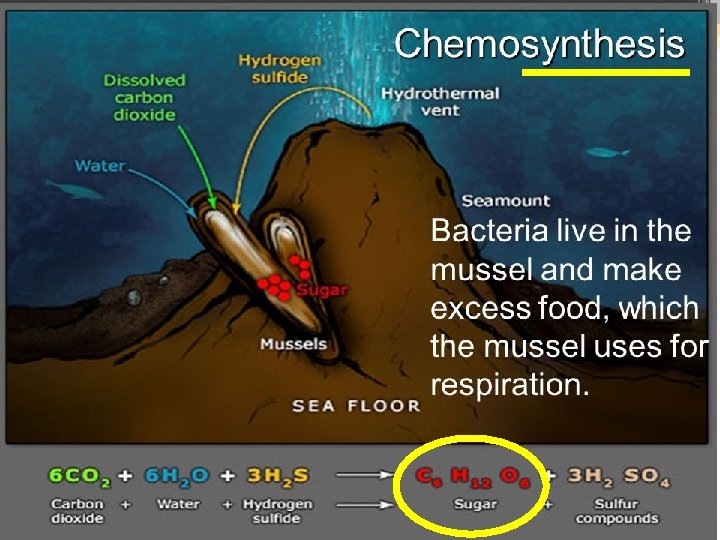
Can you see why it’s called photo. SYNTHESIS! phytoplankton & plants take 6 small molecules of carbon dioxide & water and turn it into 1 BIG glucose!

THERE’S A FEW MORE GENERAL TYPES OF CHEMICAL REACTIONS we should know. 1. OXIDATION reactions NEED O 2 as reactant A. combustion B. corrosion 2. 3. 4. 5. 6. EXOTHERMIC reactions RELEASE HEAT! ENDOTHERMIC reactions ABSORB HEAT! REPLACEMENT reactions—switcheroo!! SYNTHESIS reactions BUILDS things UP

THERE’S A FEW MORE GENERAL TYPES OF CHEMICAL REACTIONS we should know. 1. OXIDATION reactions NEED O 2 as reactant A. combustion B. corrosion 2. 3. 4. 5. 6. EXOTHERMIC reactions RELEASE HEAT! ENDOTHERMIC reactions ABSORB HEAT! REPLACEMENT reactions—switcheroo!! SYNTHESIS reactions BUILDS things UP! DECOMPOSITION reactions BREAKS DOWN
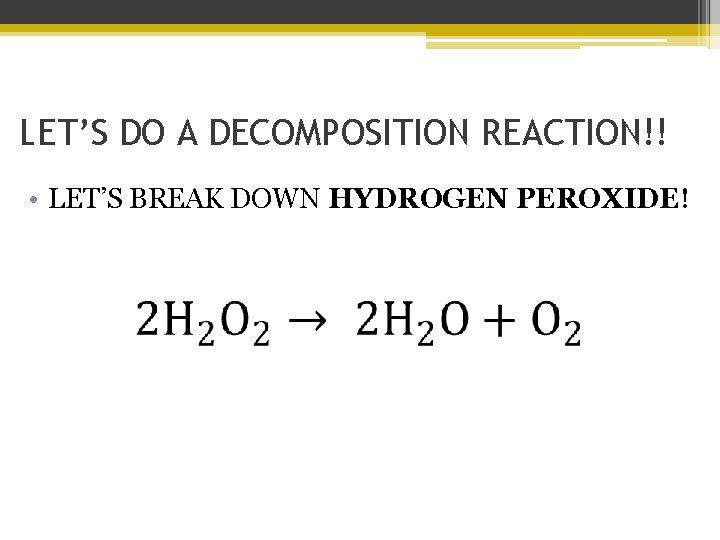
LET’S DO A DECOMPOSITION REACTION!! • LET’S BREAK DOWN HYDROGEN PEROXIDE!

How can we SPEED a reaction up? • This can take months…most decomposition reactions do.

How can we SPEED a reaction up? 1. ADD A CATALYST (substance that speeds up a reaction WITHOUT getting involved) A. chlorophyll in photosynthesis B. salt for water electrolysis C. sulfuric acid for oxidizing sugar

Catalysts are substances that speed reactions up without changing themselves… • Like a baby walker helps a newborn walk… • …BUT is unchanged.

Catalysts are substances that speed reactions up without changing themselves… • Like slippery OIL helps the engine run faster… • …BUT is unchanged.

How can we SPEED the reaction up? 1. ADD A CATALYST (substance that SPEEDS up a reaction WITHOUT getting involved) A. B. C. D. chlorophyll in photosynthesis salt for water electrolysis sulfuric acid for oxidizing sugar H 2 O 2 for speeding up our blue lab

WHAT’S HAPPENING HERE? IRON (Fe) RUSTS (CORRODES) IN WATER BUT THE RUST REACTION IS: 4 Fe + 3 O 2 2 Fe 2 O 3
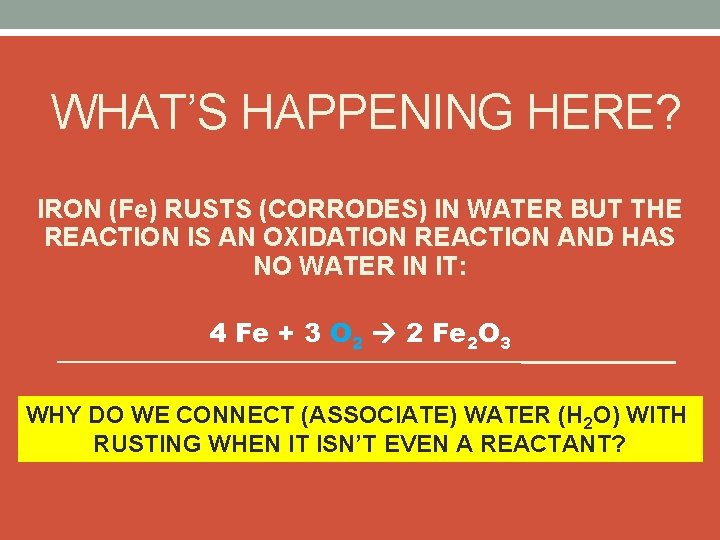
WHAT’S HAPPENING HERE? IRON (Fe) RUSTS (CORRODES) IN WATER BUT THE REACTION IS AN OXIDATION REACTION AND HAS NO WATER IN IT: 4 Fe + 3 O 2 2 Fe 2 O 3 WHY DO WE CONNECT (ASSOCIATE) WATER (H 2 O) WITH RUSTING WHEN IT ISN’T EVEN A REACTANT?

REMEMBER OUR WATER QUALITY INDICATORS? N, T or P then DO
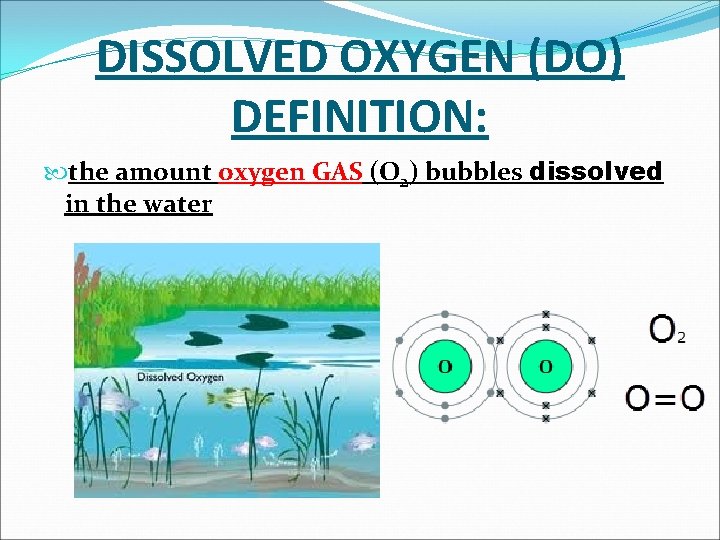
DISSOLVED OXYGEN (DO) DEFINITION: the amount oxygen GAS (O 2) bubbles dissolved in the water

GOOD DO CAUSES: MORE DISSOLVED OXYGEN LESS DISSOLVED OXYGEN turbulence (waves, rapids), REGULAR phytoplankton algae & plant growth (photosynthesis releases O 2 in water) GOOD for the water!!!

LOW DO CAUSES: LESS DISSOLVED OXYGEN higher temperatures (More O 2 escapes, possibly from high turbidity or power plants!) High nitrate levels/when algae blooms die & decomposers poop! BAD for water!!

Dissolved Oxygen RESULTS: When dissolved oxygen drops too low, FISH CAN’T BREATHE what little oxygen is there! Look for contributing causes! thermal pollution (T to high) too much nitrates high turbidity

WATER IS A CATALYST IN RUSTING! IRON (Fe) RUSTS (CORRODES) IN WATER BUT THE REACTION IS AN OXIDATION REACTION AND HAS NO WATER IN IT: H 2 O 4 Fe + 3 O 2 2 Fe 2 O 3 WATER (H 2 O) SPEEDS UP RUSTING/CORROSION BY HOLDING OXYGEN GAS ONTO THE STEEL.

How can we SPEED the reaction up? 1. ADD A CATALYST (substance that speeds up a reaction but IT DOESN’T CHANGE) A. chlorophyll in photosynthesis B. water holds O 2 onto steel to speed up oxidation

Molecules move faster when heated. The hotter it is, the quicker it COOKS.

How can we SPEED the reaction up? 1. ADD A CATALYST (substance that speeds up a reaction without getting involved) A. chlorophyll in photosynthesis B. water holds O 2 onto steel to speed up oxidation 2. INCREASE TEMPERATURE (speeds up the molecules to react faster) A. warming up our potassium permanganate & glycerin reaction

What would work faster? You want to use 3% or 30% H 2 O 2?

How can we SPEED the reaction up? 1. ADD A CATALYST (substance that speeds up a reaction without getting involved) A. chlorophyll in photosynthesis B. water holds O 2 onto steel to speed up oxidation 2. INCREASE TEMPERATURE (speeds up the molecules to react faster) A. warming up our potassium permaganate & glycerin reaction 3. INCREASE CONCENTRATION (potency of reactants can speed things along)

LAST ONE! 1. ADD A CATALYST (substance that speeds up a reaction without getting involved) A. chlorophyll in photosynthesis B. salt for water electrolysis 2. INCREASE TEMPERATURE (speeds up the molecules to react faster) A. warming up our potassium permaganate & glycerin reaction 3. INCREASE CONCENTRATION (potency of reactants can speed things along) 4. INCREASE…

WHICH REACTED FASTER YESTERDAY, THE COPPER POWDER OR COPPER PELLETS? WHY?

How can we SPEED the reaction up? 1. ADD A CATALYST (substance that speeds up a reaction without getting involved) A. chlorophyll in photosynthesis B. water holds O 2 onto steel to speed up oxidation 2. INCREASE TEMPERATURE (speeds up the molecules to react faster) A. warming up our potassium permaganate & glycerin reaction 3. INCREASE CONCENTRATION (potency of reactants can speed things along) 4. INCREASE the SURFACE AREA (smaller bits react faster) A. copper powder reacted faster than pellets!

Afterall, which works faster to get rid of your headache? GOODY’S HEADACHE POWDER BAYER ASPIRIN PILL

Goody’s powder has more surface area to react quicker than chunky pills. GOODY’S HEADACHE POWDER BAYER ASPIRIN PILL

How can we SPEED the reaction up? 1. ADD A CATALYST (substance that speeds up a reaction without getting involved) A. chlorophyll in photosynthesis B. salt for water electrolysis 2. INCREASE TEMPERATURE (speeds up the molecules to react faster) A. warming up our potassium permanganate & glycerin reaction 3. INCREASE CONCENTRATION (potency of reactants can speed things along) 4. INCREASE the SURFACE AREA (smaller bits react faster) A. copper powder reacted faster than pellets!

finish elephant toothpaste demo…

Here’s the HYDROGEN PEROXIDE DECOMPOSITION chemical reaction. 2 H 2 O 2 2 H 2 O + O 2 �We will add some dishwashing liquid as a CATALYST…

Here’s the HYDROGEN PEROXIDE DECOMPOSITION chemical reaction. 2 H 2 O 2 2 H 2 O + O 2 �We will add some dishwashing liquid as a CATALYST… �We will add a SECOND CATALYST, some YEAST.

Here’s the HYDROGEN PEROXIDE DECOMPOSITION chemical reaction. 2 H 2 O 2 2 H 2 O + O 2 �We will add some dishwashing liquid as a catalyst… �And we will add a second catalyst: yeast. �And we got water (H 2 O) & a whole bunch of oxygen gas (O 2)
- Slides: 63