Conceptual Physics The Atomic Nature of Matter Atoms










- Slides: 21

Conceptual Physics The Atomic Nature of Matter

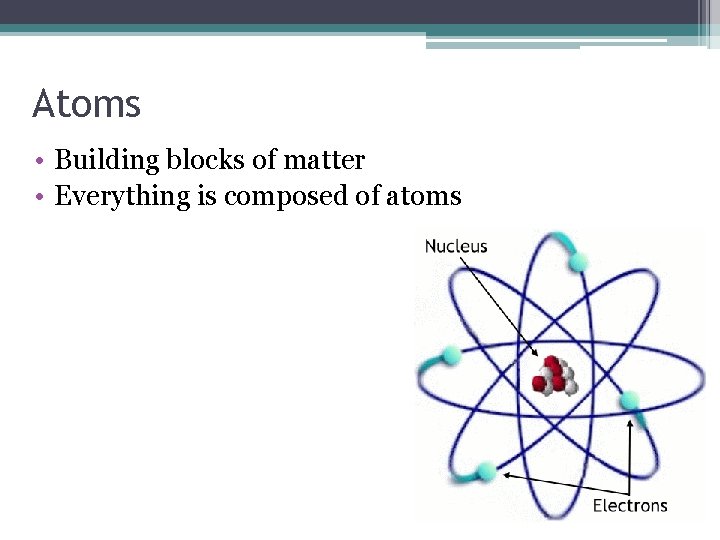
Atoms • Building blocks of matter • Everything is composed of atoms

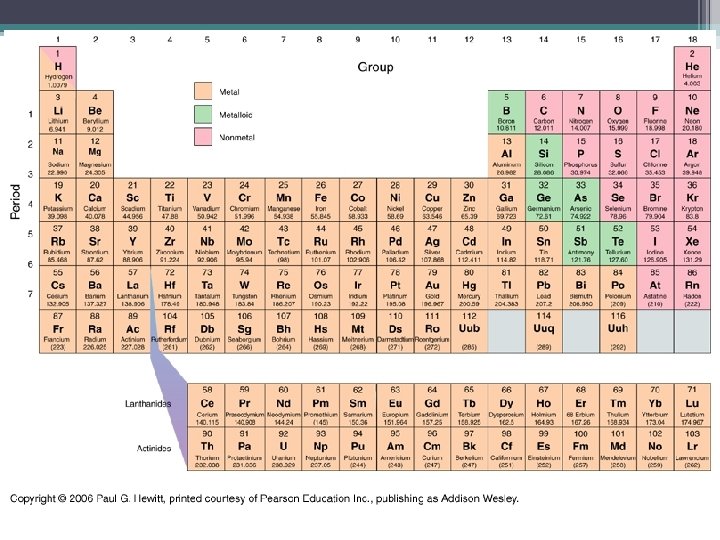
Elements • atoms of the same kind • there are 115 known elements to date • Every element is unique, and has its own set of properties • All atoms of the same element have the same # of protons.

Atoms are recyclable • the same atoms have been here since the beginning of time • Combine w/ other elements, then go back to original form and back again • You may be breathing the same air as Julius Caesar or George Washington….

Atoms are very small • Cannot view individual atoms w/ visible light • Only way to “see” atoms is w/ Scanning Tunneling Microscope • About 1023 (or 100000000000000) atoms in one thimble-full of water

Brownian Motion • Jiggling of atoms • Atoms are constantly moving • Molecular motion never stops unless a substance is at a temperature of (-273˚ C) a. k. a. absolute zero

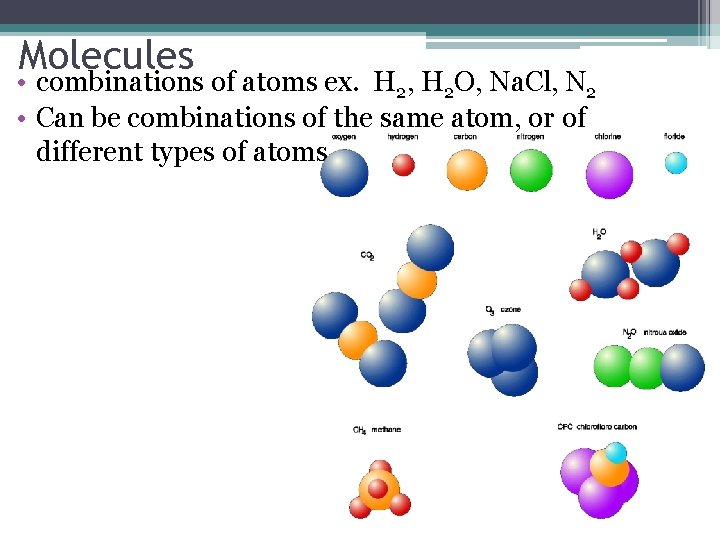
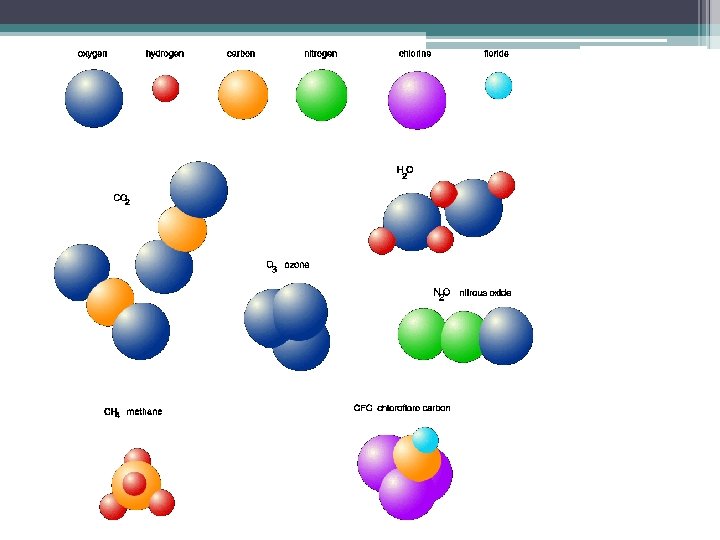
Molecules • combinations of atoms ex. H 2, H 2 O, Na. Cl, N 2 • Can be combinations of the same atom, or of different types of atoms

Compounds • combinations of atoms of different elements ex. Na. Cl, H 2 O, HCl • A compound is always a molecule, but a molecule may not necessarily be a compound (examples …. H 2 , N 2 )


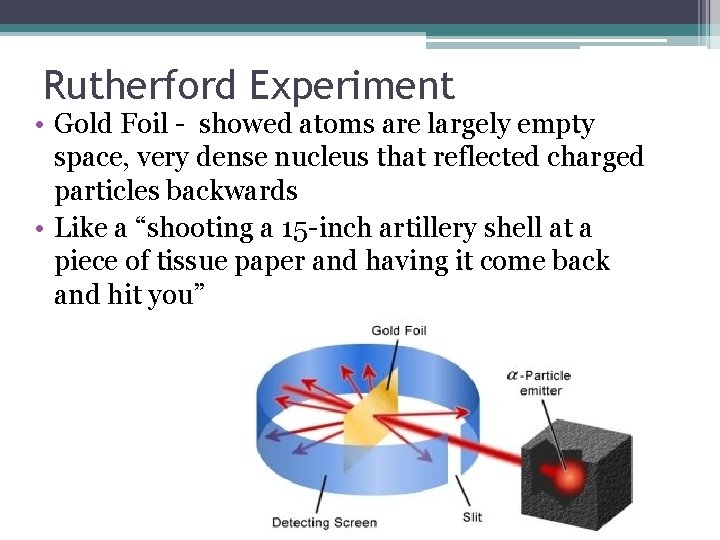
Rutherford Experiment • Gold Foil - showed atoms are largely empty space, very dense nucleus that reflected charged particles backwards • Like a “shooting a 15 -inch artillery shell at a piece of tissue paper and having it come back and hit you”

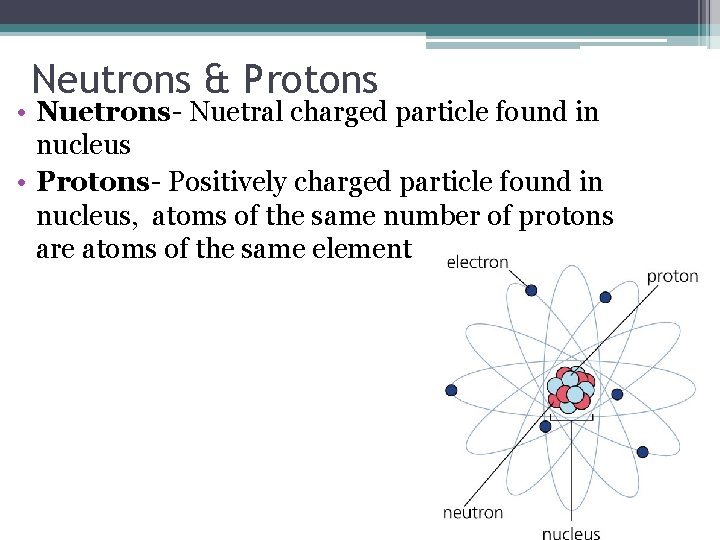
Nucleus • very dense……. Composed of Protons & Neutrons • Almost all of the mass of an atom is concentrated in the nucleus • A teaspoonful of nuclei packed together would weigh a billion tons

Neutrons & Protons • Nuetrons- Nuetral charged particle found in nucleus • Protons- Positively charged particle found in nucleus, atoms of the same number of protons are atoms of the same element

Isotopes • for a given element, the number of neutrons may vary. Isotopes of an element are atoms of the same element having different numbers of neutrons • Example - Uranium-238 (atomic mass 238) has isotope Uranium -235 ▫ This was used to make the atomic bomb dropped on Hiroshima

Electrons (e-) • negatively charged particle not found in the nucleus. • Orbits the nucleus in various energy levels. • In a neutral atom, the number of electrons will match the number of protons • # of electrons in a neutrally charged atom is equal to the # of protons, which is given with the atomic number. • Mass of 1 electron is about 1800 times less than the mass of a proton or neutron

Ions • Charged Particle ▫ Positively charged (less electrons than protons) ▫ Negatively charged (more electrons than protons) • not all atoms are neutral. This happens when the number of electrons is more than, or less than the number of protons. ▫ The number of protons never changes, ▫ however the number of electrons can change often.

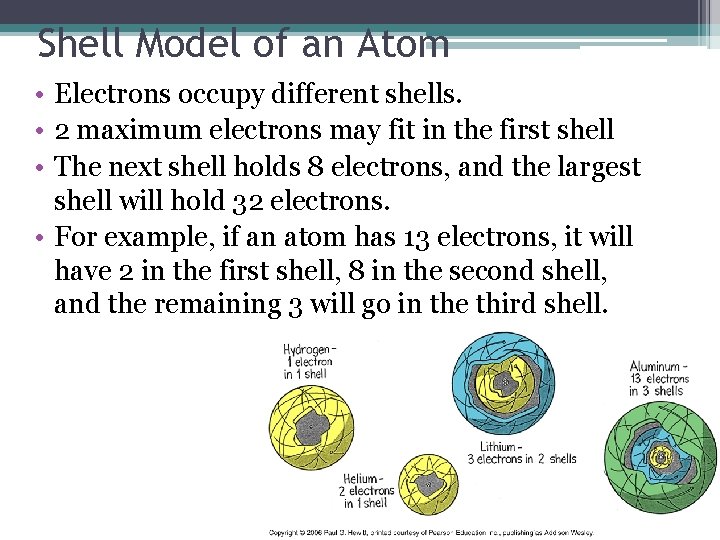
Shell Model of an Atom • Electrons occupy different shells. • 2 maximum electrons may fit in the first shell • The next shell holds 8 electrons, and the largest shell will hold 32 electrons. • For example, if an atom has 13 electrons, it will have 2 in the first shell, 8 in the second shell, and the remaining 3 will go in the third shell.

Periodic Table • chart that lists atoms by their atomic number and by their electron arrangement. • As you move from left to right on chart each element gains one proton and electron. • As you go down each column each element has one more shell filled than the element above. • Elements in the same column are in a Groups have similar properties.


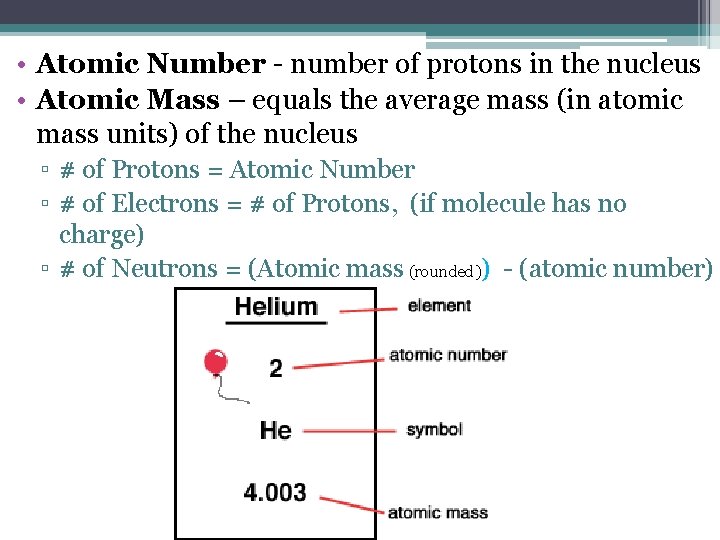
• Atomic Number - number of protons in the nucleus • Atomic Mass – equals the average mass (in atomic mass units) of the nucleus ▫ # of Protons = Atomic Number ▫ # of Electrons = # of Protons, (if molecule has no charge) ▫ # of Neutrons = (Atomic mass (rounded)) - (atomic number)

Phases of Matter • Solid, Liquid, Gas – three familiar states of matter. In all phases, atoms are in constant motion. • The more the molecules move the more fluid they become, ▫ Solid – molecules have least energy, stay fixed relative to each other ▫ Liquid- molecules have more energy, molecules move relative to one another ▫ Gas – molecules have most energy, move with large distances between each other

Plasma – the 4 th State of Matter • Consists of positive ions and free electrons. Exists only at high temperatures. • What sun and stars are made of. Can be seen here on Earth in the glowing gas of fluorescent lamps.