Conceptual Physical Science 5 th Edition Chapter 7



























- Slides: 64

Conceptual Physical Science 5 th Edition Chapter 7: HEAT TRANSFER AND CHANGE OF PHASE © 2012 Pearson Education, Inc.

This lecture will help you understand: • • • Conduction Convection Radiation Newton’s Law of Cooling Climate Change and the Greenhouse Effect Heat Transfer and Change of Phase Boiling Melting and Freezing Energy and Change of Phase © 2012 Pearson Education, Inc.

Heat Transfer Processes of thermal energy transfer: • Conduction • Convection • Radiation © 2012 Pearson Education, Inc.

Conduction • Transfer of internal energy by electron and molecular collisions within a substance © 2012 Pearson Education, Inc.

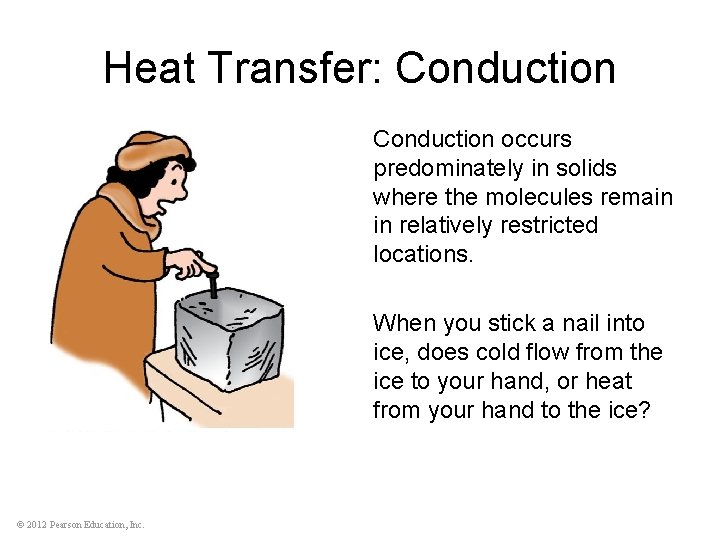
Heat Transfer: Conduction occurs predominately in solids where the molecules remain in relatively restricted locations. When you stick a nail into ice, does cold flow from the ice to your hand, or heat from your hand to the ice? © 2012 Pearson Education, Inc.

Heat Transfer: Conduction CHECK YOUR NEIGHBOR If you hold one end of a metal bar against a piece of ice, the end in your hand will soon become cold. Does cold flow from the ice to your hand? A. B. C. D. Yes. In some cases, yes. No. In some cases, no. © 2012 Pearson Education, Inc.

Heat Transfer: Conduction CHECK YOUR ANSWER If you hold one end of a metal bar against a piece of ice, the end in your hand will soon become cold. Does cold flow from the ice to your hand? A. B. C. D. Yes. In some cases, yes. No. In some cases, no. Explanation: Cold does not flow from the ice to your hand. Heat flows from your hand to the ice. The metal is cold to your touch, because you are transferring heat to the metal. © 2012 Pearson Education, Inc.

Conduction Insulation • Doesn’t prevent the flow of internal energy • Slows the rate at which internal energy flows Example: Rock wool or fiberglass between walls slows the transfer of internal energy from a warm house to a cool exterior in winter, and the reverse in summer © 2012 Pearson Education, Inc.

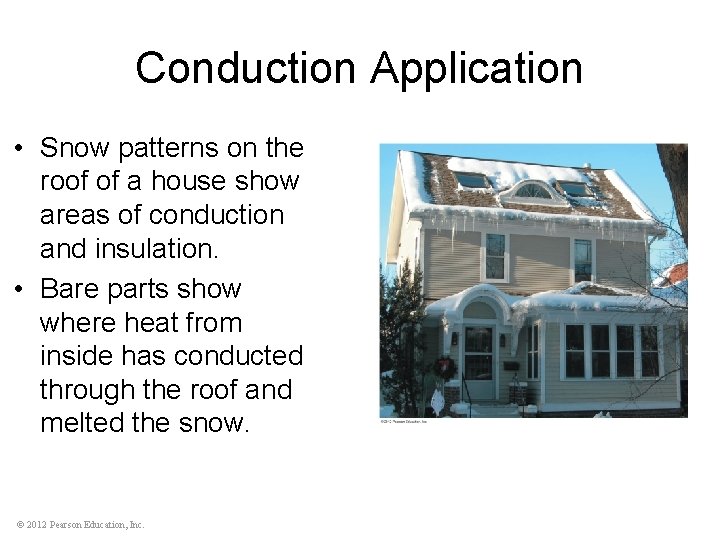
Conduction Application • Snow patterns on the roof of a house show areas of conduction and insulation. • Bare parts show where heat from inside has conducted through the roof and melted the snow. © 2012 Pearson Education, Inc.

Energy Transfer CHECK YOUR NEIGHBOR When thermal insulation, such as spun glass or rock wool, is placed beneath the roof of a house, then in cold weather the insulation will A. B. C. D. create heat to warm the house. keep the cold from coming through the roof. slow the flow of heat from inside the house to the outside. stop the flow of heat from inside the house to the outside. © 2012 Pearson Education, Inc.

Energy Transfer CHECK YOUR ANSWER When thermal insulation, such as spun glass or rock wool, is placed beneath the roof of a house, then in cold weather the insulation will A. B. C. D. create heat to warm the house. keep the cold from coming through the roof. slow the flow of heat from inside the house to the outside. stop the flow of heat from inside the house to the outside. Explanation: No insulation can stop heat flow. Insulation only slows it. (A fortune awaits the inventor who can come up with the “perfect” insulator!) © 2012 Pearson Education, Inc.

Heat Transfer: Conduction Good conductors: • Composed of atoms with “loose” outer electrons • Known as poor insulators • Examples—all metals to varying degrees Poor conductors: • Delay the transfer of heat • Known as good insulators • Examples—wood, wool, straw, paper, Styrofoam, cork, liquid, gases, air, or materials with trapped air © 2012 Pearson Education, Inc.

Conduction Dramatic example: © 2012 Pearson Education, Inc. Author John Suchocki walks barefoot without burning his feet on red-hot coals, due to poor conduction between the coals and his feet

Conduction Dramatic example: © 2012 Pearson Education, Inc. Author John Suchocki walks barefoot without burning his feet on red-hot coals, due to poor conduction between the coals and his feet.

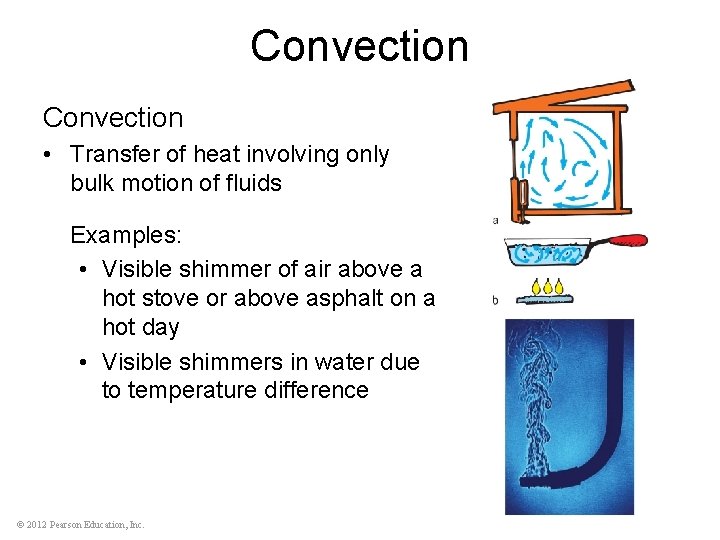
Convection • Transfer of heat involving only bulk motion of fluids Examples: • Visible shimmer of air above a hot stove or above asphalt on a hot day • Visible shimmers in water due to temperature difference © 2012 Pearson Education, Inc.

Convection Cooling by expansion • Opposite to the warming that occurs when air is compressed Example: The “cloudy” region above hot steam issuing from the nozzle of a pressure cooker is cool to the touch (a combination of air expansion and mixing with cooler surrounding air). Careful, the part at the nozzle that you can’t see is steam—ouch! © 2012 Pearson Education, Inc.

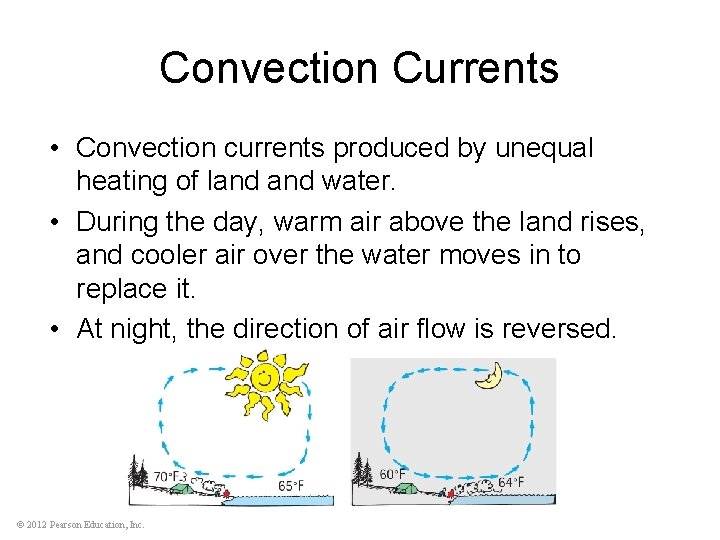
Convection Currents • Convection currents produced by unequal heating of land water. • During the day, warm air above the land rises, and cooler air over the water moves in to replace it. • At night, the direction of air flow is reversed. © 2012 Pearson Education, Inc.

Convection Reason warm air rises • Warm air expands, becomes less dense, and is buoyed upward • Air rises until its density equals that of the surrounding air Example: Smoke from a campfire rises and blends with the surrounding cool air. © 2012 Pearson Education, Inc.

Heat Transfer: Convection CHECK YOUR NEIGHBOR Although warm air rises, why are mountaintops cold and snow covered, while the valleys below are relatively warm and green? A. B. C. D. Warm air cools when rising. There is a thick insulating blanket of air above valleys. Both of the above. None of the above. © 2012 Pearson Education, Inc.

Heat Transfer: Convection CHECK YOUR ANSWER Although warm air rises, why are mountaintops cold and snow covered, while the valleys below are relatively warm and green? A. B. C. D. Warm air cools when rising. There is a thick insulating blanket of air above valleys. Both of the above. None of the above. Explanation: Earth’s atmosphere acts as a blanket, which for one important thing, keeps Earth from freezing at nighttime. © 2012 Pearson Education, Inc.

Radiation • Transfer of energy via electromagnetic waves that can travel through empty space © 2012 Pearson Education, Inc.

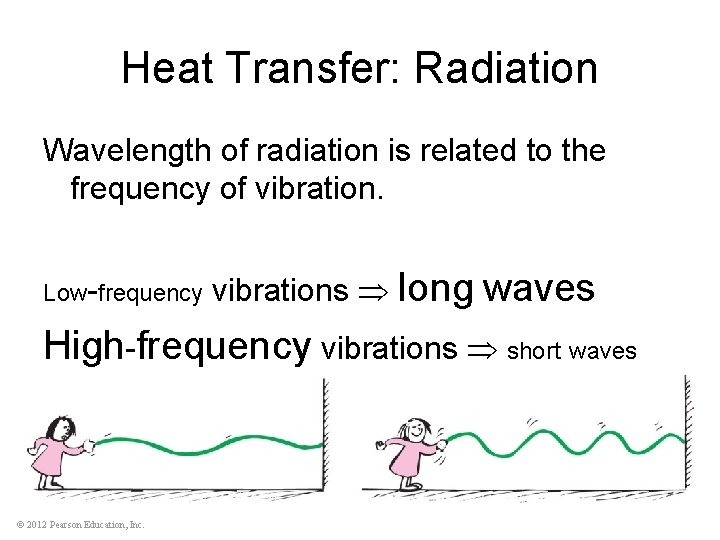
Heat Transfer: Radiation Wavelength of radiation is related to the frequency of vibration. Low-frequency vibrations long waves High-frequency vibrations short waves © 2012 Pearson Education, Inc.

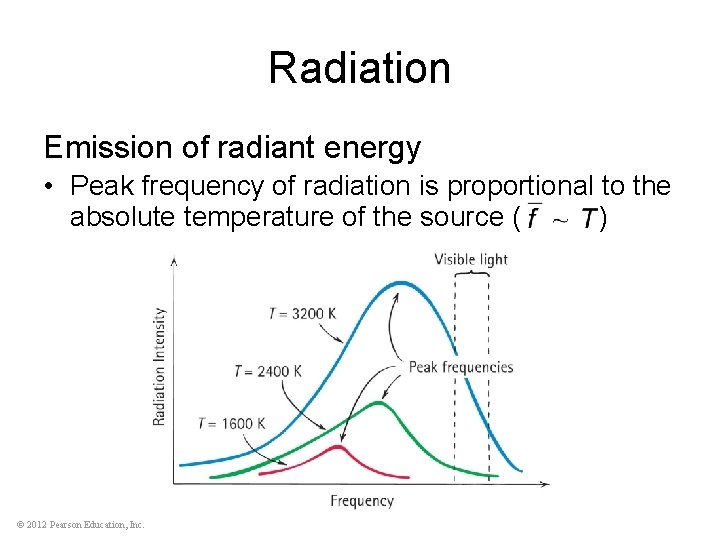
Radiation Emission of radiant energy • Every object above absolute zero radiates • From the Sun’s surface comes light, or solar radiation • From the Earth’s surface is terrestrial radiation in the form of infrared waves below our threshold of sight © 2012 Pearson Education, Inc.

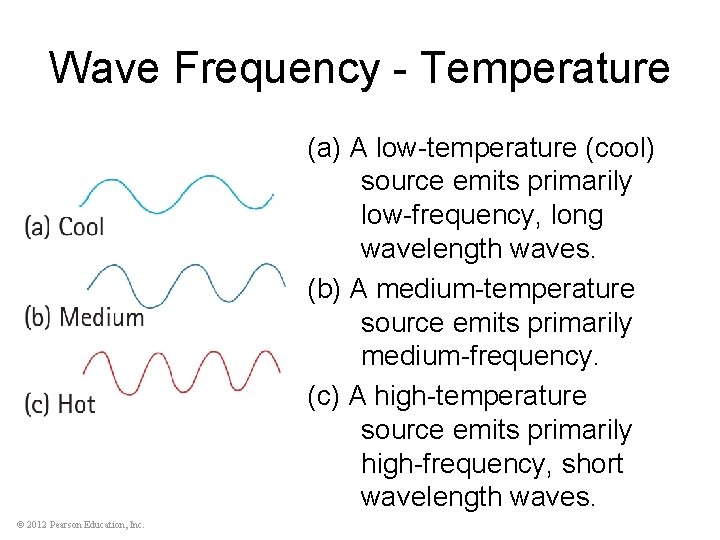
Wave Frequency - Temperature (a) A low-temperature (cool) source emits primarily low-frequency, long wavelength waves. (b) A medium-temperature source emits primarily medium-frequency. (c) A high-temperature source emits primarily high-frequency, short wavelength waves. © 2012 Pearson Education, Inc.

Radiation Emission of radiant energy • Peak frequency of radiation is proportional to the absolute temperature of the source ( ) © 2012 Pearson Education, Inc.

Emission and Absorption The surface of any material both absorbs and emits radiant energy. When a surface absorbs more energy than it emits, it is a net absorber, and temperature tends to rise. When a surface emits more energy than it absorbs, it is a net emitter, and temperature tends to fall. © 2012 Pearson Education, Inc.

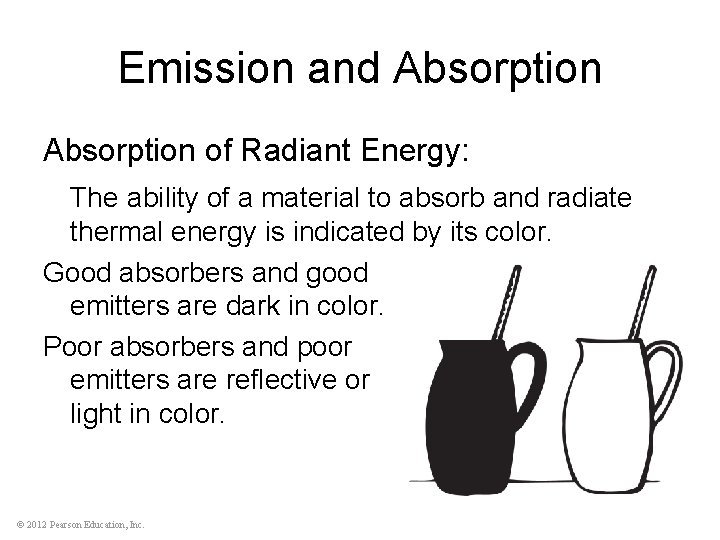
Emission and Absorption of Radiant Energy: The ability of a material to absorb and radiate thermal energy is indicated by its color. Good absorbers and good emitters are dark in color. Poor absorbers and poor emitters are reflective or light in color. © 2012 Pearson Education, Inc.

Emission and Absorption Whether a surface is a net absorber or net emitter depends on whether its temperature is above or below that of its surroundings. A surface hotter than its surroundings will be a net emitter and tends to cool. A surface colder than its surroundings will be a net absorber and tends to warm. © 2012 Pearson Education, Inc.

Emission and Absorption CHECK YOUR NEIGHBOR If a good absorber of radiant energy were a poor emitter, its temperature compared with its surroundings would be A. B. C. D. lower. higher. unaffected. None of the above. © 2012 Pearson Education, Inc.

Emission and Absorption CHECK YOUR ANSWER If a good absorber of radiant energy were a poor emitter, its temperature compared with its surroundings would be A. B. C. D. lower. higher. unaffected. None of the above. Explanation: If a good absorber were not also a good emitter, there would be a net absorption of radiant energy, and the temperature of a good absorber would remain higher than the temperature of the surroundings. Nature is not so! © 2012 Pearson Education, Inc.

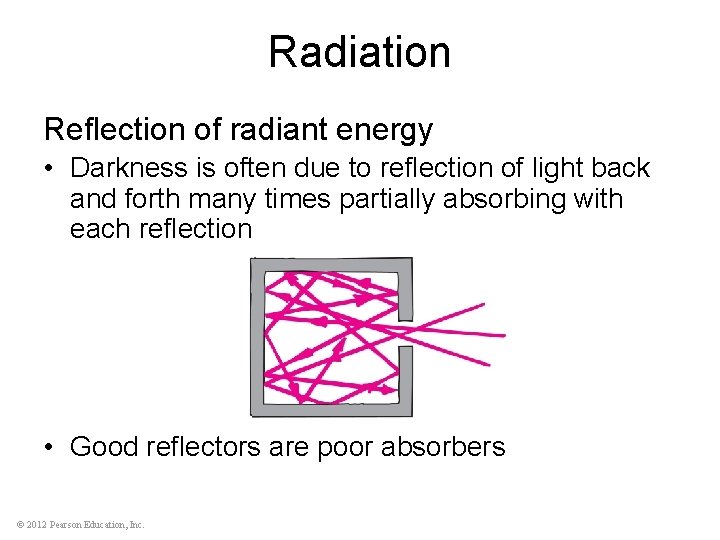
Radiation Reflection of radiant energy • Darkness is often due to reflection of light back and forth many times partially absorbing with each reflection • Good reflectors are poor absorbers © 2012 Pearson Education, Inc.

Absorption of Radiant Energy CHECK YOUR NEIGHBOR Which is the better statement? A. B. C. D. A black object absorbs energy well. An object that absorbs energy well is black. Both say the same thing, so both are equivalent. Both are untrue. © 2012 Pearson Education, Inc.

Absorption of Radiant Energy CHECK YOUR ANSWER Which is the better statement? A. B. C. D. A black object absorbs energy well. An object that absorbs energy well is black. Both say the same thing, so both are equivalent. Both are untrue. Explanation: This is a cause-and-effect question. The color black doesn’t draw in and absorb energy. It’s the other way around—any object that does draw in and absorb energy, will, by consequence, be black in color. © 2012 Pearson Education, Inc.

Emission of Radiant Energy CHECK YOUR NEIGHBOR Which of the following does NOT emit radiation? A. B. C. D. A lit fluorescent lamp. A lit incandescent lamp. A burned out incandescent lamp. None of the above. © 2012 Pearson Education, Inc.

Emission of Radiant Energy CHECK YOUR ANSWER Which of the following does NOT emit radiation? A. B. C. D. A lit fluorescent lamp. A lit incandescent lamp. A burned out incandescent lamp. None of the above. Explanation: Everything continually emits radiation—and everything continually absorbs radiation. When emission is greater than absorption, temperature of the emitter drops. When absorption is greater than emission, temperature increases. Everything is emitting and absorbing radiation continually. That’s right—everything! © 2012 Pearson Education, Inc.

Newton’s Law of Cooling • Approximately proportional to the temperature difference T between the object and its surroundings • In short: Rate of cooling ~ T Examples: • Hot apple pie cools more quickly in a freezer than if left on the kitchen table • Warmer house more quickly leaks thermal energy to the outside than a cooler house © 2012 Pearson Education, Inc.

Newton’s Law of Cooling (continued) • Applies to rate of warming – Object cooler than its surroundings warms up at a rate proportional to T Example: Frozen food warm quicker in a warm room than in a cold room © 2012 Pearson Education, Inc.

Newton’s Law of Cooling CHECK YOUR NEIGHBOR It is commonly thought that a can of beverage will cool faster in the coldest part of a refrigerator. Knowledge of Newton’s law of cooling A. B. C. D. supports this knowledge. shows this knowledge is false. may or may not support this knowledge. may or may not contradict this knowledge. © 2012 Pearson Education, Inc.

Newton’s Law of Cooling CHECK YOUR ANSWER It is commonly thought that a can of beverage will cool faster in the coldest part of a refrigerator. Knowledge of Newton’s law of cooling A. B. C. D. supports this knowledge. shows this knowledge is false. may or may not support this knowledge. may or may not contradict this knowledge. © 2012 Pearson Education, Inc.

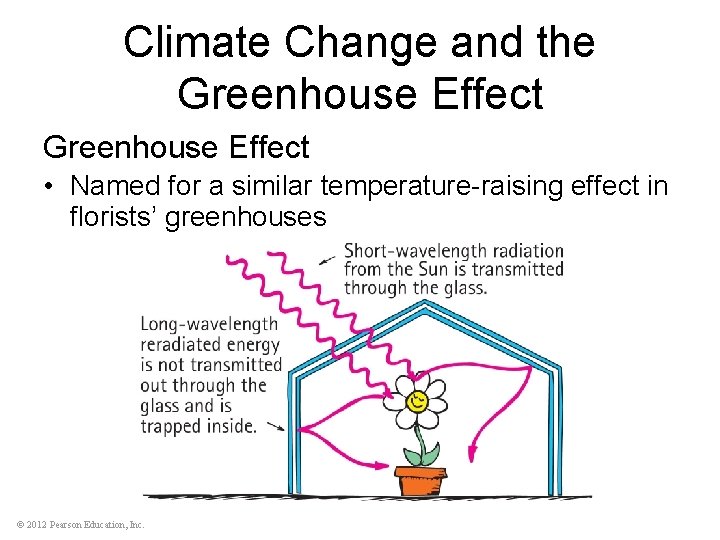
Climate Change and the Greenhouse Effect • Named for a similar temperature-raising effect in florists’ greenhouses © 2012 Pearson Education, Inc.

Climate Change and the Greenhouse Effect Understanding the greenhouse effect requires two concepts: • All things radiate at a frequency (and therefore wavelength) that depends on the temperature of the emitting object • transparency of things depends on the wavelength of radiation © 2012 Pearson Education, Inc.

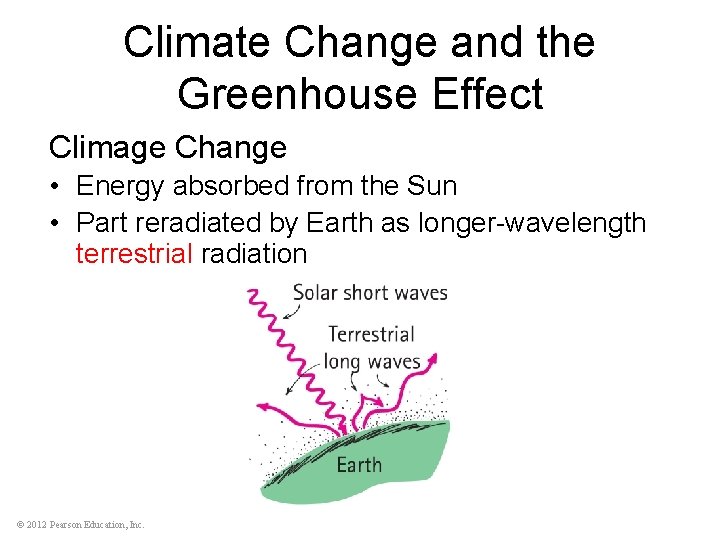
Climate Change and the Greenhouse Effect Climage Change • Energy absorbed from the Sun • Part reradiated by Earth as longer-wavelength terrestrial radiation © 2012 Pearson Education, Inc.

Climate Change and the Greenhouse Effect Climate Change (continued) • Terrestrial radiation absorbed by atmospheric gases and re-emitted as long-wavelength terrestrial radiation back to Earth • Reradiated energy unable to escape, so warming of Earth occurs • Long-term effects on climate are of present concern © 2012 Pearson Education, Inc.

Climate Change and the Greenhouse Effect CHECK YOUR NEIGHBOR If Earth radiated more energy than it absorbs from the Sun, Earth’s average temperature would A. B. C. D. decrease. increase. likely not change. None of these. © 2012 Pearson Education, Inc.

Climate Change and the Greenhouse Effect CHECK YOUR ANSWER If Earth radiated more energy than it absorbs from the Sun, Earth’s average temperature would A. B. C. D. decrease. increase. likely not change. None of these. Explanation: The Sun emits short-wavelength radiation and the Earth absorbs it. Earth emits long-wavelength radiation, and we have an energy balance that determines Earth’s temperature. If Earth radiates more than it absorbs, then like any system, its temperature would decrease. That’s the physics! © 2012 Pearson Education, Inc.

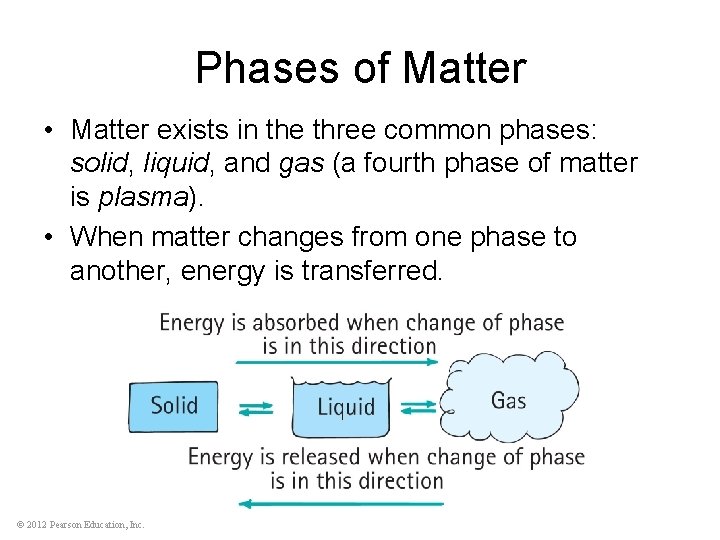
Phases of Matter • Matter exists in the three common phases: solid, liquid, and gas (a fourth phase of matter is plasma). • When matter changes from one phase to another, energy is transferred. © 2012 Pearson Education, Inc.

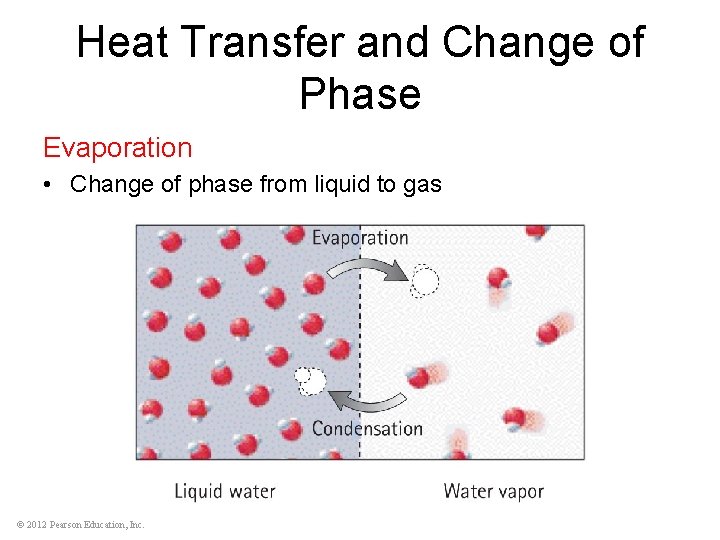
Heat Transfer and Change of Phase Evaporation • Change of phase from liquid to gas © 2012 Pearson Education, Inc.

Heat Transfer and Change of Phase Evaporation process • Molecules in liquid move randomly at various speeds, continually colliding with one another • Some molecules gain kinetic energy while others lose kinetic energy during collision • Some energetic molecules escape from the liquid and become gas • Average kinetic energy of the remaining molecules in the liquid decreases, resulting in cooler water © 2012 Pearson Education, Inc.

Evaporation Application • Pigs have no sweat glands and therefore cannot cool by the evaporation of perspiration. • Instead, they wallow in mud to cool themselves. © 2012 Pearson Education, Inc.

Heat Transfer and Change of Phase Sublimation • Form of phase change directly from solid to gas Examples: • dry ice (solid carbon dioxide molecules) • mothballs • frozen water © 2012 Pearson Education, Inc.

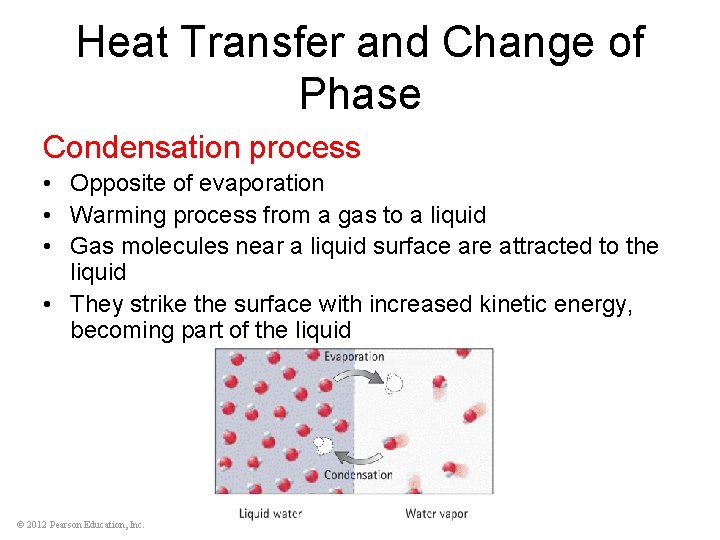
Heat Transfer and Change of Phase Condensation process • Opposite of evaporation • Warming process from a gas to a liquid • Gas molecules near a liquid surface are attracted to the liquid • They strike the surface with increased kinetic energy, becoming part of the liquid © 2012 Pearson Education, Inc.

Condensation Application • If you’re chilly outside the shower stall, step back inside and be warmed by the condensation of the excess water vapor in the shower • Evaporation cools you. Condensation warms you! © 2012 Pearson Education, Inc.

Evaporation-Condensation Toy The toy drinking bird operates by the evaporation of ether inside its body and by the evaporation of water from the outer surface of its head. The lower body contains liquid ether, which evaporates at room temperature. (a) vaporizes, (b) creates pressure, ether goes up the tube, (c) tips over, condensed ether runs back to body. (d) each pivot wets the head and the cycle repeats. © 2012 Pearson Education, Inc.

Energy Transfer CHECK YOUR NEIGHBOR When a liquid changes phase to a gas, it A. B. C. D. absorbs energy. emits energy. neither absorbs nor emits energy. becomes more conducting. © 2012 Pearson Education, Inc.

Energy Transfer CHECK YOUR ANSWER When a liquid changes phase to a gas, it A. B. C. D. absorbs energy. emits energy. neither absorbs nor emits energy. becomes more conducting. © 2012 Pearson Education, Inc.

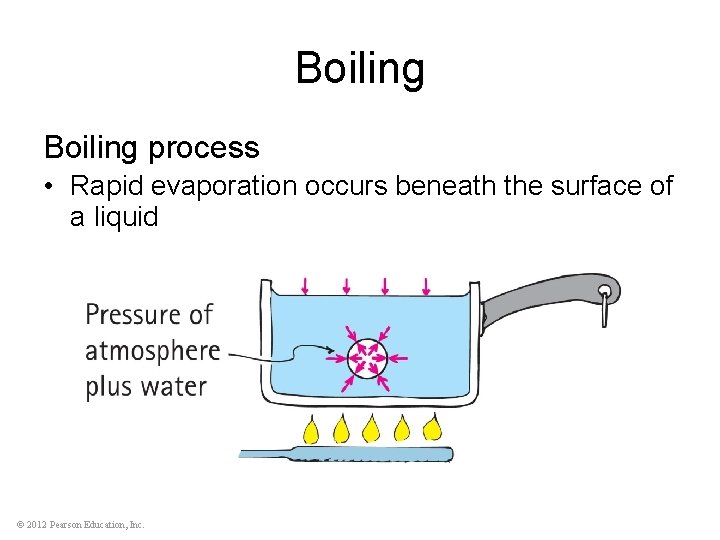
Boiling process • Rapid evaporation occurs beneath the surface of a liquid © 2012 Pearson Education, Inc.

Boiling process (continued) • evaporation beneath the surface forms vapor bubbles • bubbles rise to the surface • if vapor pressure in the bubble is less than the surrounding pressure, then the bubbles collapse • hence, bubbles don’t form at temperatures below boiling point when vapor pressure is insufficient © 2012 Pearson Education, Inc.

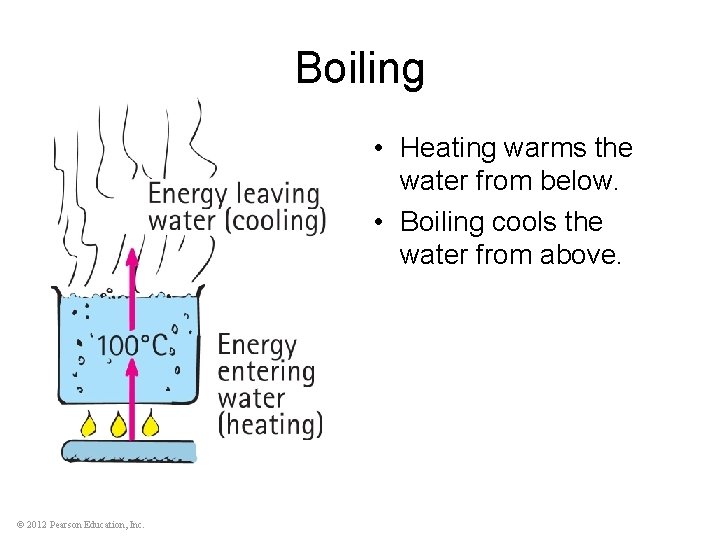
Boiling • Heating warms the water from below. • Boiling cools the water from above. © 2012 Pearson Education, Inc.

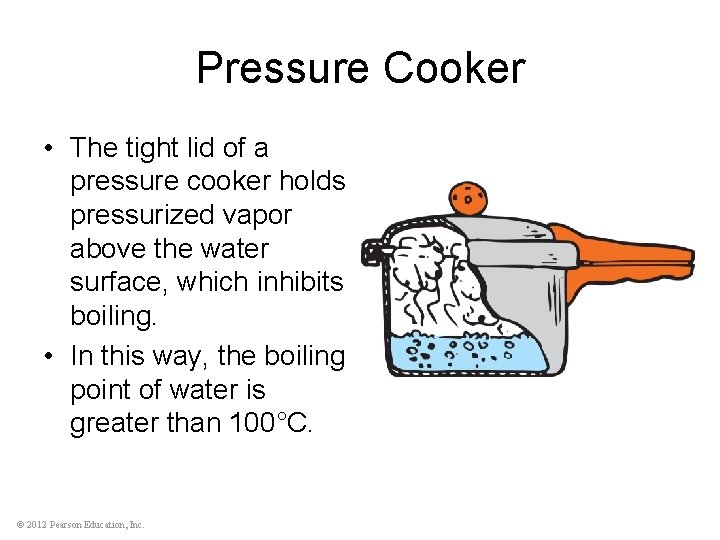
Pressure Cooker • The tight lid of a pressure cooker holds pressurized vapor above the water surface, which inhibits boiling. • In this way, the boiling point of water is greater than 100°C. © 2012 Pearson Education, Inc.

Boiling CHECK YOUR NEIGHBOR When a liquid is brought to a boil, the boiling process tends to A. B. C. D. resist a further change of phase. heat the liquid. cool the liquid. radiate energy from the system. © 2012 Pearson Education, Inc.

Boiling CHECK YOUR ANSWER When a liquid is brought to a boil, the boiling process tends to A. B. C. D. resist a further change of phase. heat the liquid. cool the liquid. radiate energy from the system. © 2012 Pearson Education, Inc.

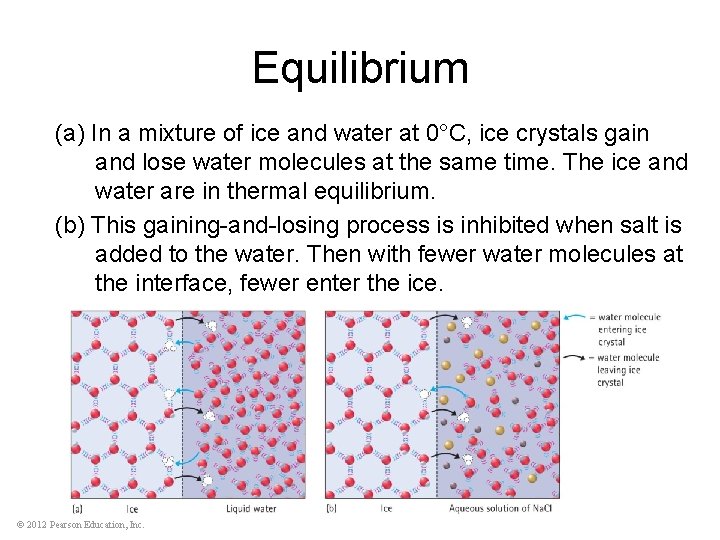
Equilibrium (a) In a mixture of ice and water at 0°C, ice crystals gain and lose water molecules at the same time. The ice and water are in thermal equilibrium. (b) This gaining-and-losing process is inhibited when salt is added to the water. Then with fewer water molecules at the interface, fewer enter the ice. © 2012 Pearson Education, Inc.

Can Crunch! CHECK YOUR NEIGHBOR A spectacular classroom (or home) demo is placing a sodapop can with a bit of water in it on a hot stove. Soon steam comes from the opening. Invert the can into a bath of water and atmospheric pressure crushes the can. The key to this dramatic whopping of the can is mainly A. B. C. D. temperature reduction of the steam in the can. rapid condensation of steam in the can. reduced pressure due to cooling of steam in the can. increase in atmospheric pressure on the can. © 2012 Pearson Education, Inc.

Can Crunch! CHECK YOUR ANSWER A spectacular classroom (or home) demo is placing a sodapop can with a bit of water in it on a hot stove. Soon steam comes from the opening. Invert the can into a bath of water and atmospheric pressure crushes the can. The key to this dramatic whopping of the can is mainly A. B. C. D. temperature reduction of the steam in the can. rapid condensation of steam in the can. reduced pressure due to cooling of steam in the can. increase in atmospheric pressure on the can. Explanation: Quick reduction in pressure occurs as steam molecules rapidly condense upon the water in the bath (they don’t condense on the hot inner metal walls). With lowered pressure inside, the outside atmospheric pressure produces the whop! Similarly, the condensation cycle of steam turbines reduces pressure on the back side of turbine blades. Greater steam pressure on the front side of the blades turns the turbine. © 2012 Pearson Education, Inc.