Conceptual Chemistry UNIT 4 CHEMICAL EQUATIONS REACTIONS Objective

Conceptual Chemistry UNIT 4 – CHEMICAL EQUATIONS & REACTIONS

Objective 1 Read and express information given in chemical formulas.
Chemical Formula Shows the type and number of atoms present in a substance.

Chemical Formula • In the chemical formula, subscripts show many atoms are in the compound. C 6 H 10 N 2 O 2 C=6 H= 10 N= 2 O= 2

Chemical Formula The large numbers in front of the chemical formula, called coefficients, show the number of particles. 3 C 6 H 10 N 2 O 2 = 3 total

Practice Problems: How atoms of each element are in the following? 1. CH 4 2. Al. Cl 3 3. 4 H 2 O 4. 10 Fe 3 P 2 5. 6 Zn(NO 3)2 6. Al 2(SO 3)3

Objective 2 Balance chemical equations.

Chemical Equation Reactants Products Ex: 2 KCl. O 3 2 KCl + 3 O 2 Reactant = KCl. O 3 Products = KCl + O 2
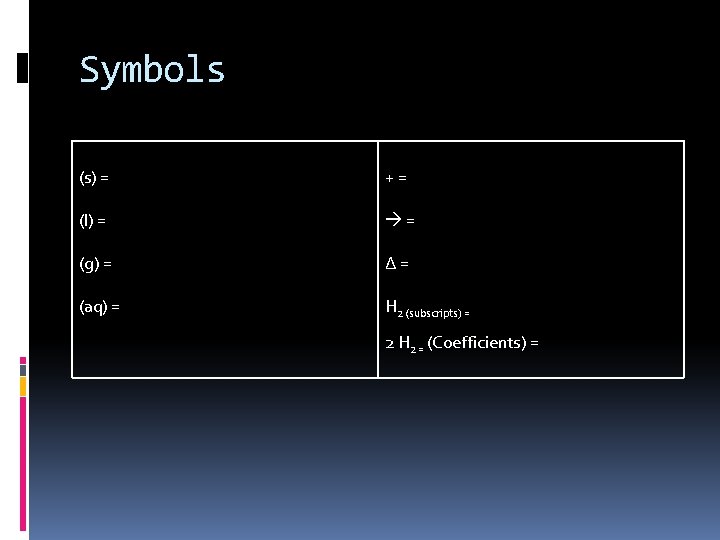
Symbols (s) = += (l) = = (g) = ∆= (aq) = H 2 (subscripts) = 2 H 2 = (Coefficients) =
Balancing Equations The law of conservation of mass states that matter cannot be created or destroyed. The number of atoms reacting must equal the number of atoms produced. A chemical change rearranges these atoms into new substances.

To balance equations, follow these steps: 1. Count atoms on each side of the arrow. They should be equal. If not, you need to balance them. 2. Balance both sides by using coefficients. (They multiply through everything inside of that compound. ) NEVER change subscripts! 3. Double check work.
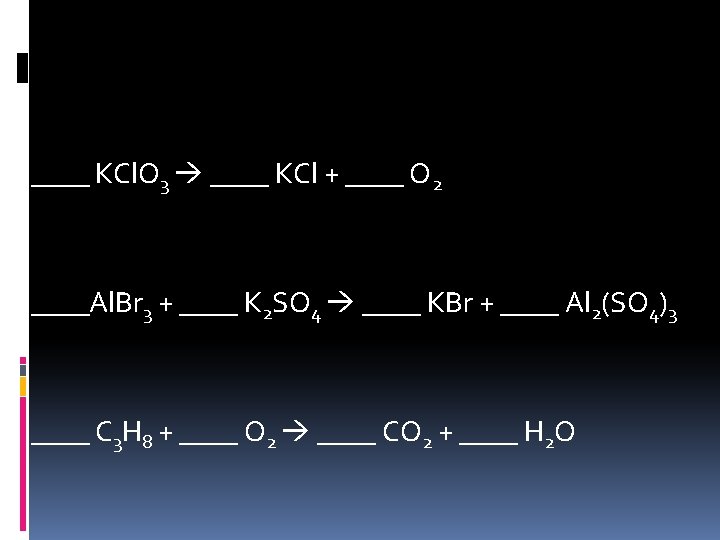
Practice Problems ____ KCl. O 3 ____ KCl + ____ O 2 ____Al. Br 3 + ____ K 2 SO 4 ____ KBr + ____ Al 2(SO 4)3 ____ C 3 H 8 + ____ O 2 ____ CO 2 + ____ H 2 O

Objective 3 Describe chemical reactions and calculate percent yield.
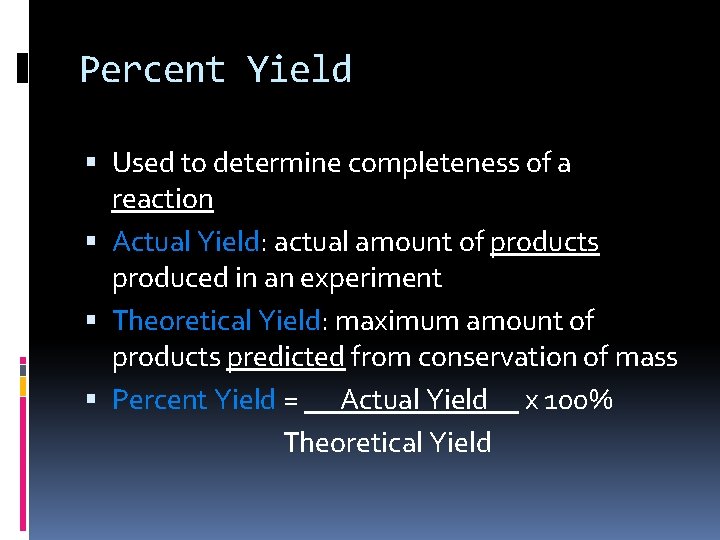
Percent Yield Used to determine completeness of a reaction Actual Yield: actual amount of products produced in an experiment Theoretical Yield: maximum amount of products predicted from conservation of mass Percent Yield = Actual Yield x 100% Theoretical Yield

Practice Problem When a measured amount of hydrogen gas reacted with a measured amount of oxygen gas, 2. 5 g of water was produced. The expected amount was 3. 0 g. What was the percent yield?
- Slides: 15