Concepts of Matter and Energy Matter Anything that





- Slides: 22

Concepts of Matter and Energy • Matter: Anything that has mass and occupies space; made of atoms and molecules – States: solid, liquid and gas • Energy: Ability to do work or put matter into motion – Kinetic: energy is actually doing work (moving matter using muscles to lift) – Potential: Stored or inactive energy (like in an ATP bond)

Types of energy: – Chemical energy: Stored in bonds, such as in high energy foods (complex carbohydrates); energy is released when bond is broken. – Electrical energy: Movement of charged particles, such as the sodium/potassium pump used for nerve impulses. – Mechanical energy: Directly moves matter, such as muscles moving a load. – Radiant energy: Traveling in waves, such as the light energy that stimulates the retina in the back of your eye.

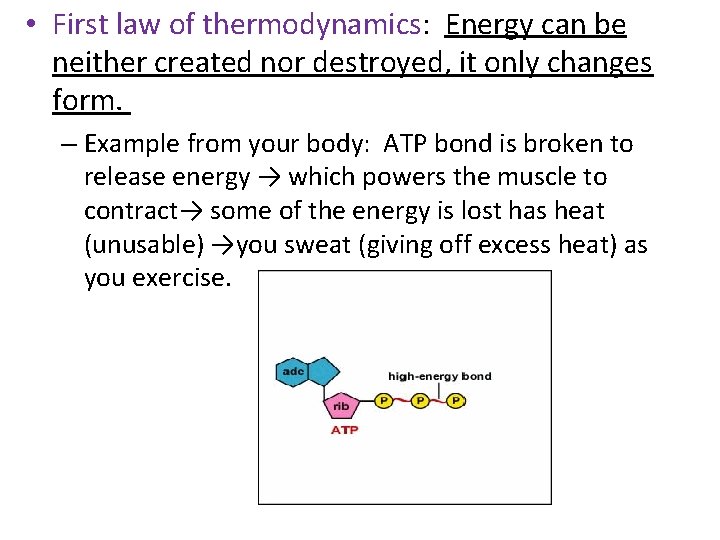
• First law of thermodynamics: Energy can be neither created nor destroyed, it only changes form. – Example from your body: ATP bond is broken to release energy → which powers the muscle to contract→ some of the energy is lost has heat (unusable) →you sweat (giving off excess heat) as you exercise.

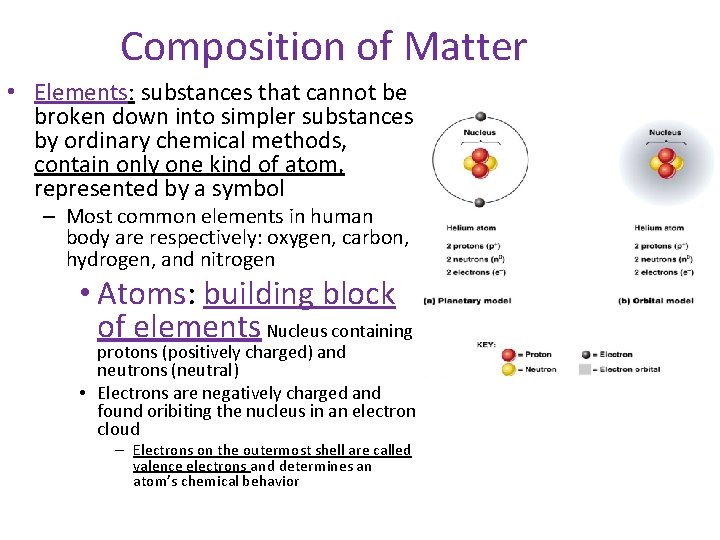
Composition of Matter • Elements: substances that cannot be broken down into simpler substances by ordinary chemical methods, contain only one kind of atom, represented by a symbol – Most common elements in human body are respectively: oxygen, carbon, hydrogen, and nitrogen • Atoms: building block of elements Nucleus containing protons (positively charged) and neutrons (neutral) • Electrons are negatively charged and found oribiting the nucleus in an electron cloud – Electrons on the outermost shell are called valence electrons and determines an atom’s chemical behavior

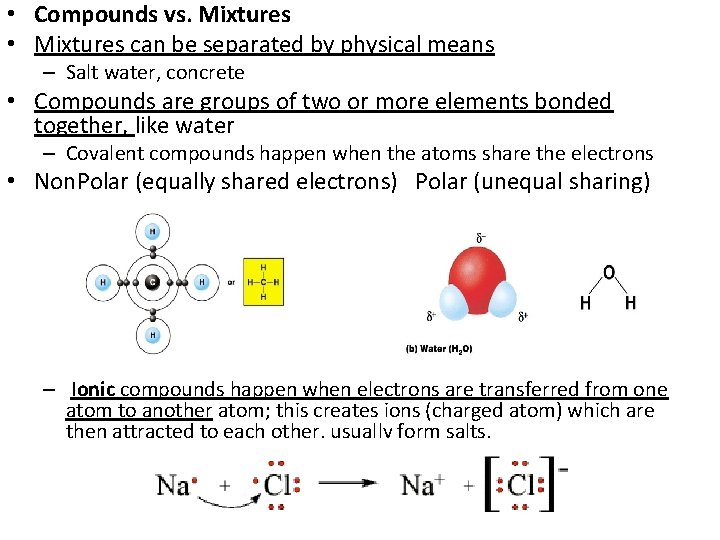
• Compounds vs. Mixtures • Mixtures can be separated by physical means – Salt water, concrete • Compounds are groups of two or more elements bonded together, like water – Covalent compounds happen when the atoms share the electrons • Non. Polar (equally shared electrons) Polar (unequal sharing) – Ionic compounds happen when electrons are transferred from one atom to another atom; this creates ions (charged atom) which are then attracted to each other, usually form salts.

Inorganic Compounds • Compounds that do not contain carbon – Water, salts, acids and bases • Water is the most abundant inorganic compound in your body (60 -80% of your body weight) – High heat capacity- prevents sudden changes in body temperature – Polar- makes water an excellent solvent allowing it to transport nutrients, gases and wastes – Chemical reactions- water is an important reactant in hydrolysis reactions (breaking down large molecules in our food) – Cushioning- water is protective; cerebrospinal fluid, amniotic fluid

• Salts, acids and bases are all called ELECTROLYTES (they ionize and then dissociate in water to conduct an electrical current) • Salts are compounds formed from ionic bonding. – When dissolved in body fluids easily separate into ions (charged particles), and are called electrolytes • Important for metabolic processes such as sodium/potassium pump for conducting nerve impulses; Iron found on hemoglobin in red blood cells to transport oxygen. TOP six ions in body 1. ) Sodium - Na+ 2. )Potassium - K+ 3. ) Calcium - Ca++ 4. ) Magnesium - Mg++ 5. ) Chloride - Cl 6. ) Hydrogen - H+

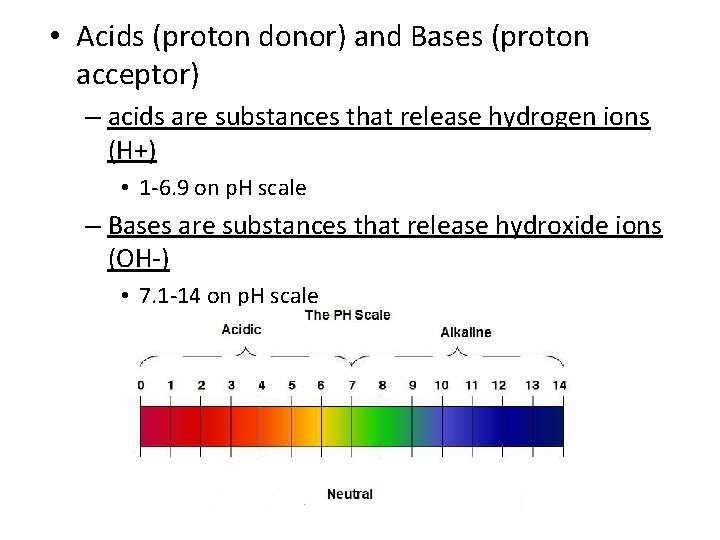
• Acids (proton donor) and Bases (proton acceptor) – acids are substances that release hydrogen ions (H+) • 1 -6. 9 on p. H scale – Bases are substances that release hydroxide ions (OH-) • 7. 1 -14 on p. H scale

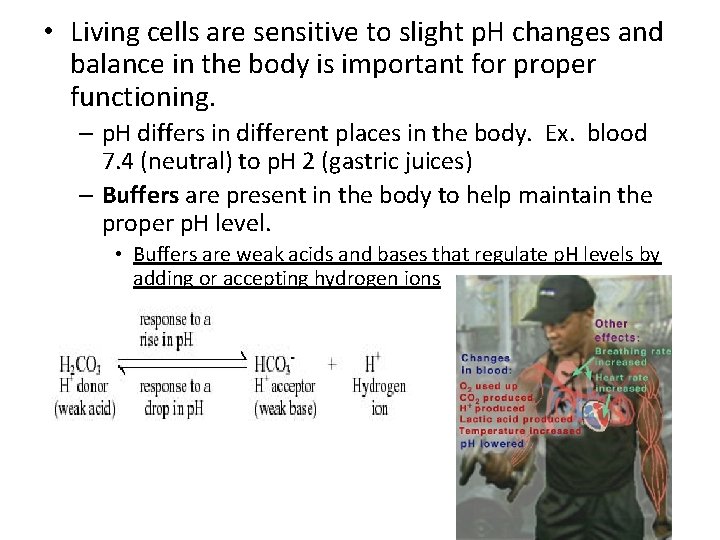
• Living cells are sensitive to slight p. H changes and balance in the body is important for proper functioning. – p. H differs in different places in the body. Ex. blood 7. 4 (neutral) to p. H 2 (gastric juices) – Buffers are present in the body to help maintain the proper p. H level. • Buffers are weak acids and bases that regulate p. H levels by adding or accepting hydrogen ions

Organic Compounds • Carbon containing compounds (must have carbon AND hydrogen) • Organic compounds such as carbohydrates, lipids, nucleic acids and proteins are important to our body

The “Core Four” Organic Compounds • Carbohydrates • Lipids • Nucleic Acids • Proteins

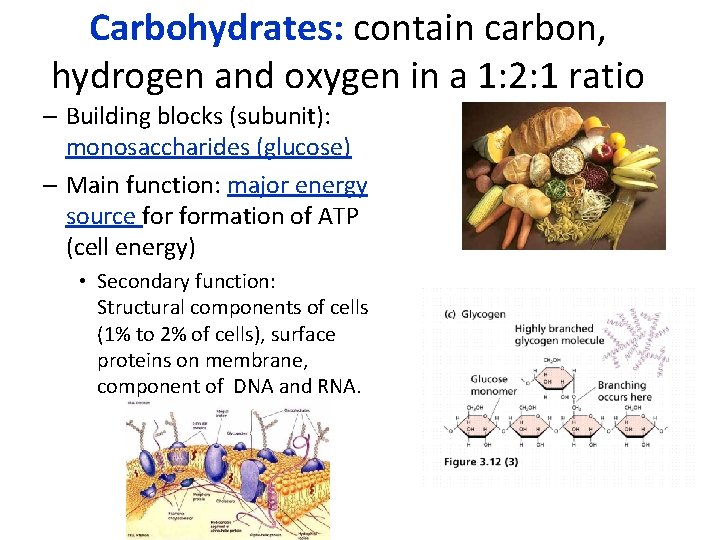
Carbohydrates: contain carbon, hydrogen and oxygen in a 1: 2: 1 ratio – Building blocks (subunit): monosaccharides (glucose) – Main function: major energy source formation of ATP (cell energy) • Secondary function: Structural components of cells (1% to 2% of cells), surface proteins on membrane, component of DNA and RNA.

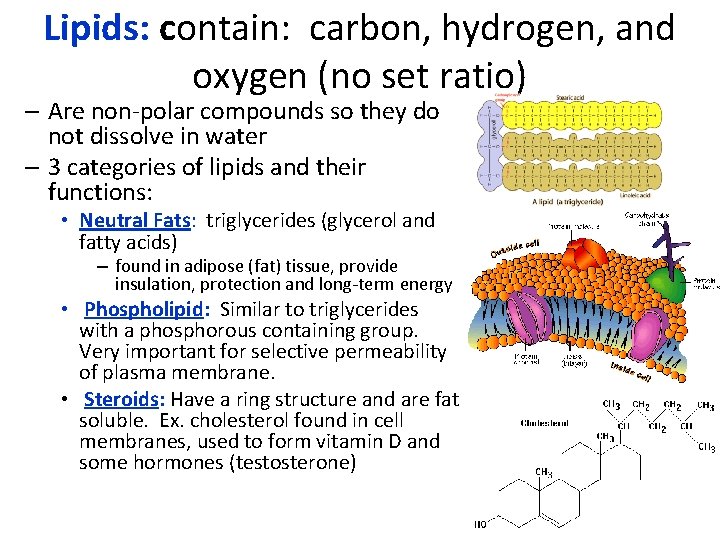
Lipids: contain: carbon, hydrogen, and oxygen (no set ratio) – Are non-polar compounds so they do not dissolve in water – 3 categories of lipids and their functions: • Neutral Fats: triglycerides (glycerol and fatty acids) – found in adipose (fat) tissue, provide insulation, protection and long-term energy • Phospholipid: Similar to triglycerides with a phosphorous containing group. Very important for selective permeability of plasma membrane. • Steroids: Have a ring structure and are fat soluble. Ex. cholesterol found in cell membranes, used to form vitamin D and some hormones (testosterone)

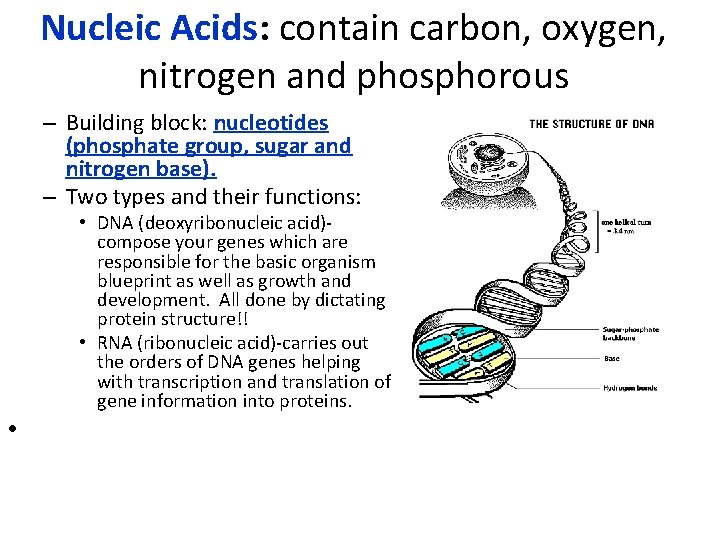
Nucleic Acids: contain carbon, oxygen, nitrogen and phosphorous – Building block: nucleotides (phosphate group, sugar and nitrogen base). – Two types and their functions: • DNA (deoxyribonucleic acid)compose your genes which are responsible for the basic organism blueprint as well as growth and development. All done by dictating protein structure!! • RNA (ribonucleic acid)-carries out the orders of DNA genes helping with transcription and translation of gene information into proteins. •

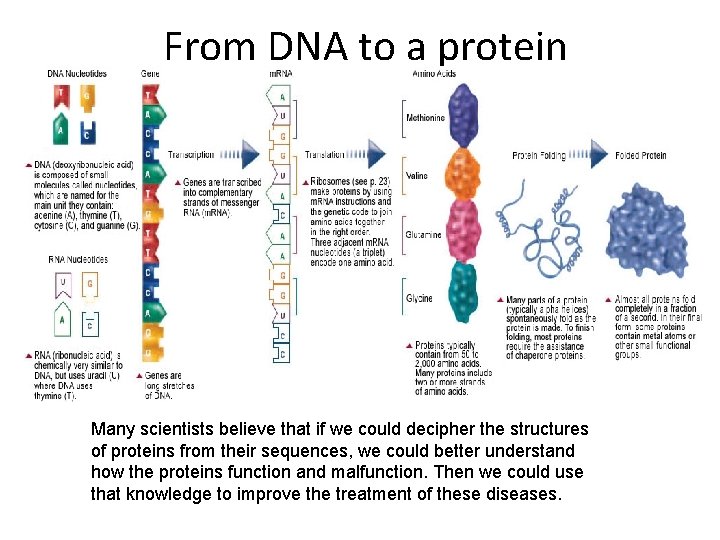
From DNA to a protein Many scientists believe that if we could decipher the structures of proteins from their sequences, we could better understand how the proteins function and malfunction. Then we could use that knowledge to improve the treatment of these diseases.

Food • When we eat, food is in the form of POLYMERS. • Our body uses food in the form of MONOMERS. • This is why our food needs to be digested: Polymers Monomers

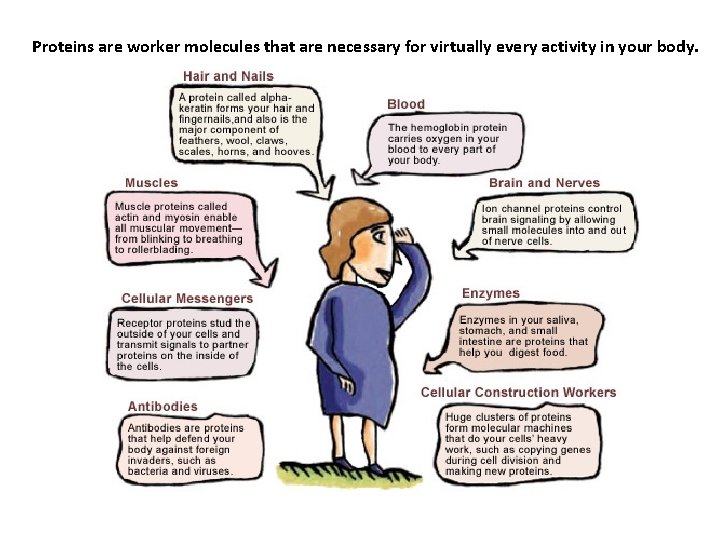
Proteins are worker molecules that are necessary for virtually every activity in your body.

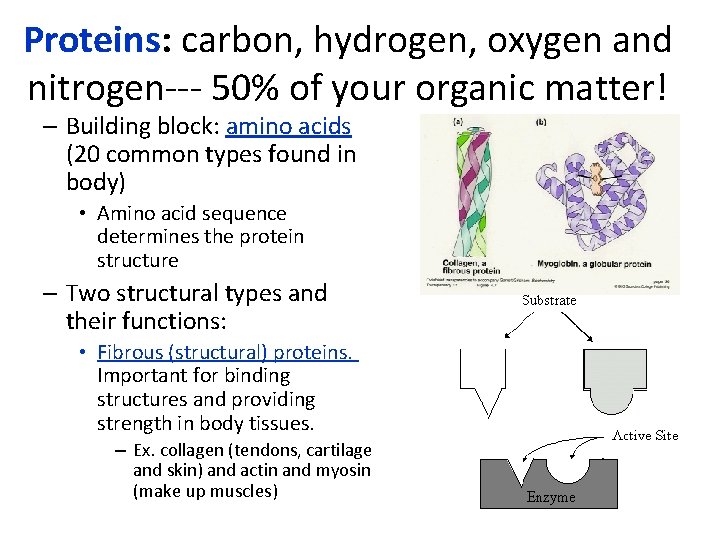
Proteins: carbon, hydrogen, oxygen and nitrogen--- 50% of your organic matter! – Building block: amino acids (20 common types found in body) • Amino acid sequence determines the protein structure – Two structural types and their functions: • Fibrous (structural) proteins. Important for binding structures and providing strength in body tissues. – Ex. collagen (tendons, cartilage and skin) and actin and myosin (make up muscles)

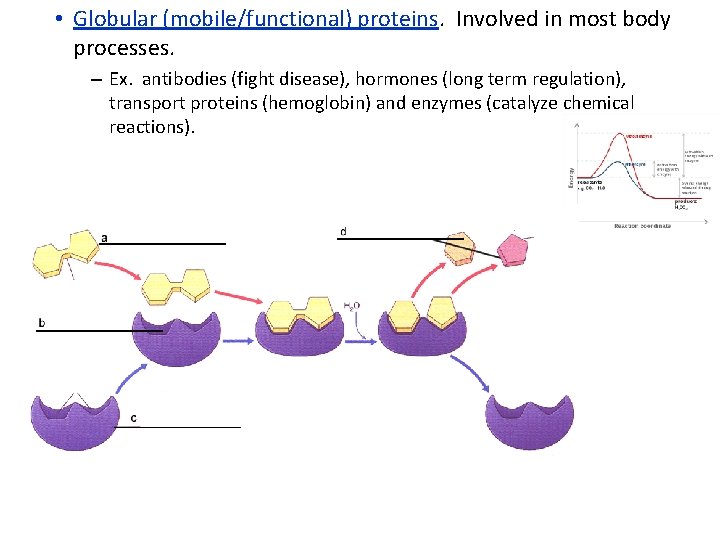
• Globular (mobile/functional) proteins. Involved in most body processes. – Ex. antibodies (fight disease), hormones (long term regulation), transport proteins (hemoglobin) and enzymes (catalyze chemical reactions).

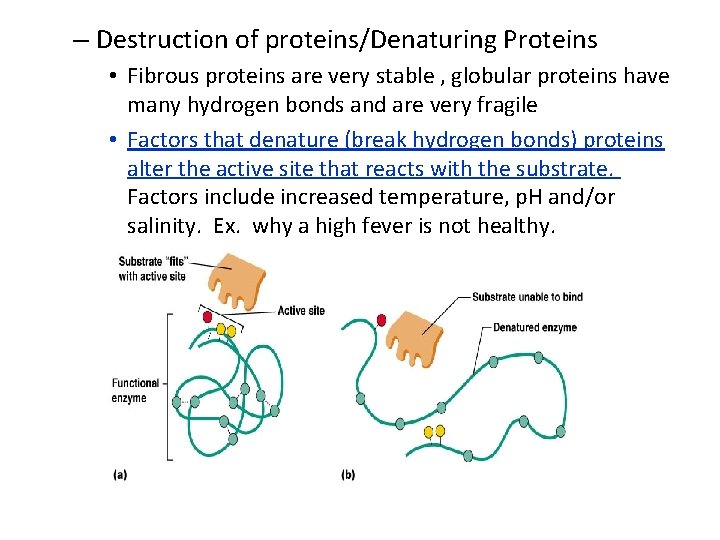
– Destruction of proteins/Denaturing Proteins • Fibrous proteins are very stable , globular proteins have many hydrogen bonds and are very fragile • Factors that denature (break hydrogen bonds) proteins alter the active site that reacts with the substrate. Factors include increased temperature, p. H and/or salinity. Ex. why a high fever is not healthy.

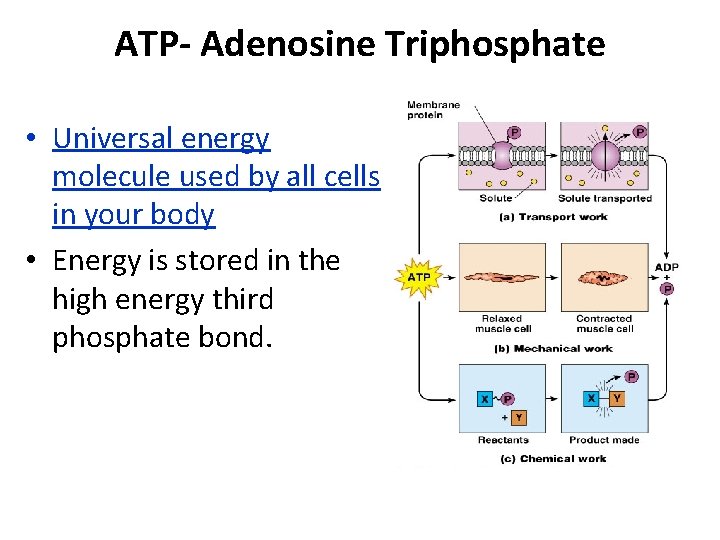
ATP- Adenosine Triphosphate • Universal energy molecule used by all cells in your body • Energy is stored in the high energy third phosphate bond.

How does our body digest organic molecules? • Where do these molecules go and what does our body use them for? • http: //kitses. com/animati on/swfs/digestion. swf