Concept Check What is the change in internal

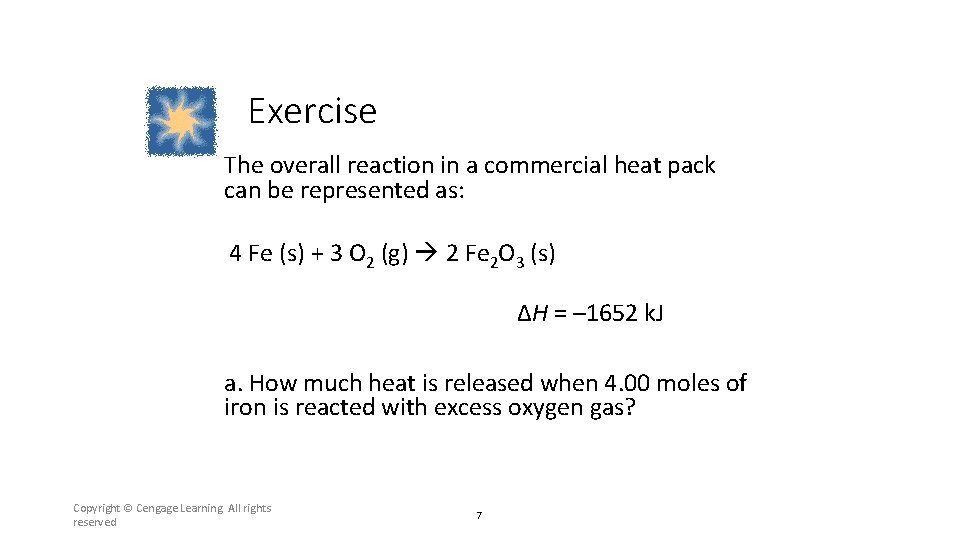


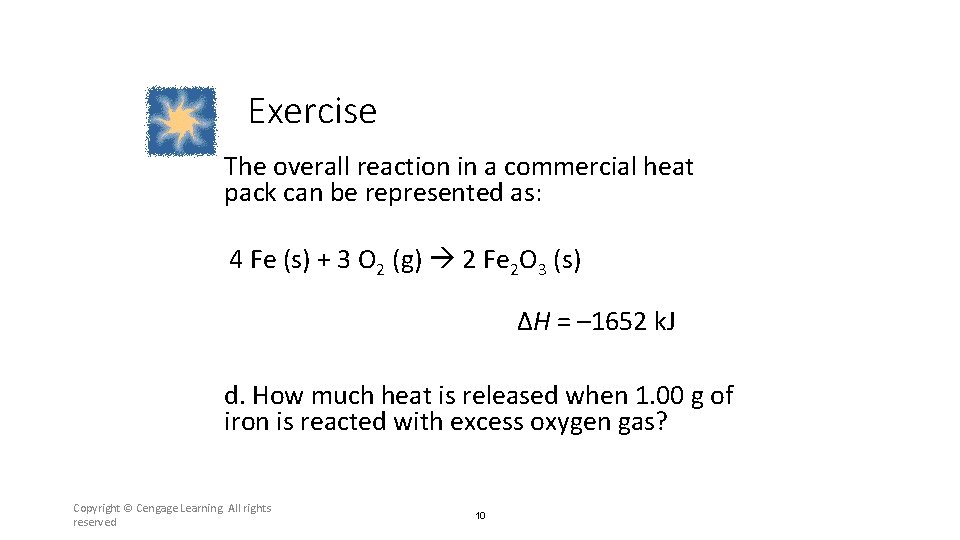


















- Slides: 51


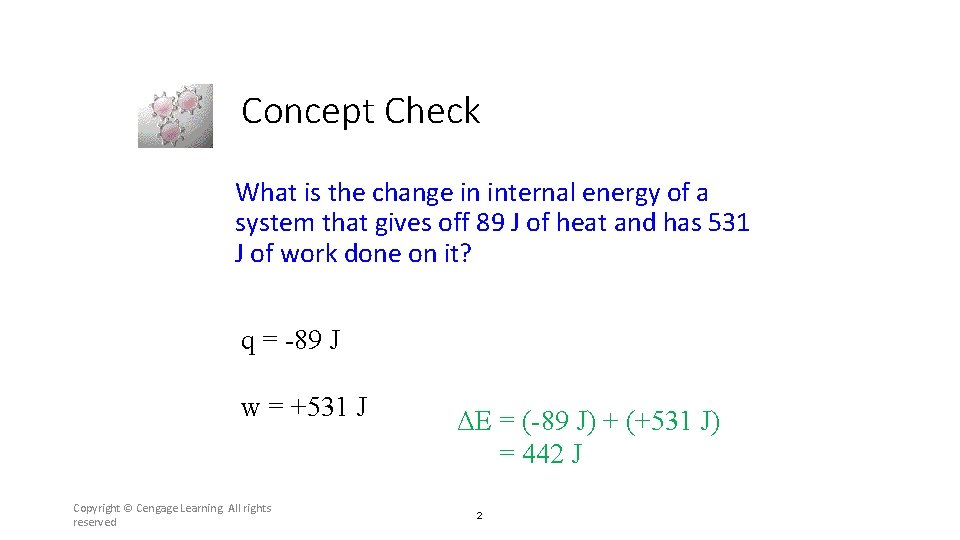
Concept Check What is the change in internal energy of a system that gives off 89 J of heat and has 531 J of work done on it? q = -89 J w = +531 J Copyright © Cengage Learning. All rights reserved ΔE = (-89 J) + (+531 J) = 442 J 2

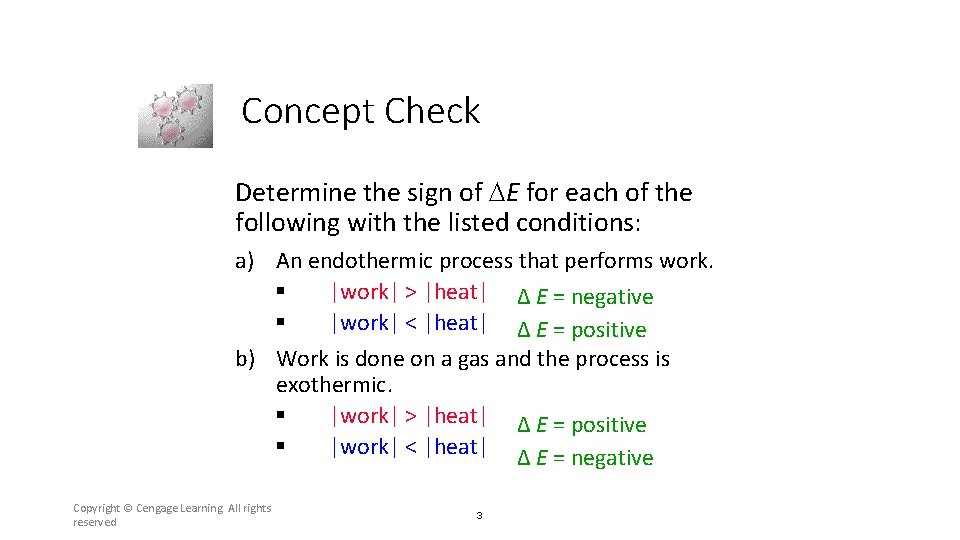
Concept Check Determine the sign of E for each of the following with the listed conditions: a) An endothermic process that performs work. § |work| > |heat| Δ E = negative § |work| < |heat| Δ E = positive b) Work is done on a gas and the process is exothermic. § |work| > |heat| Δ E = positive § |work| < |heat| Δ E = negative Copyright © Cengage Learning. All rights reserved 3

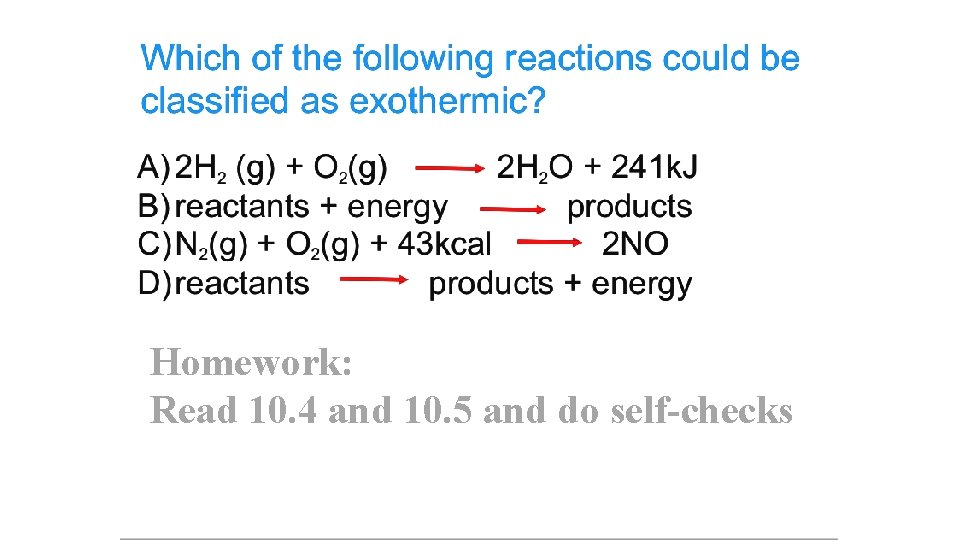
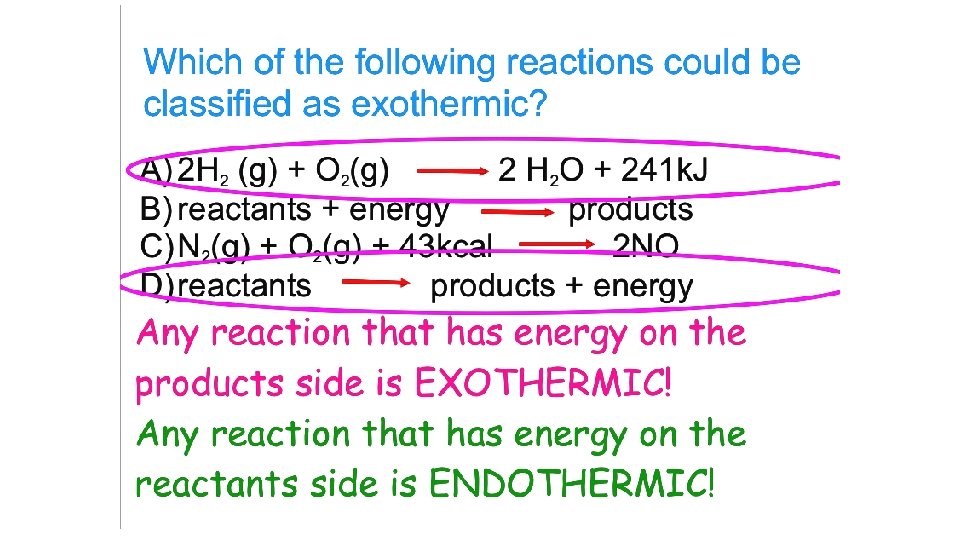
Homework: Read 10. 4 and 10. 5 and do self-checks


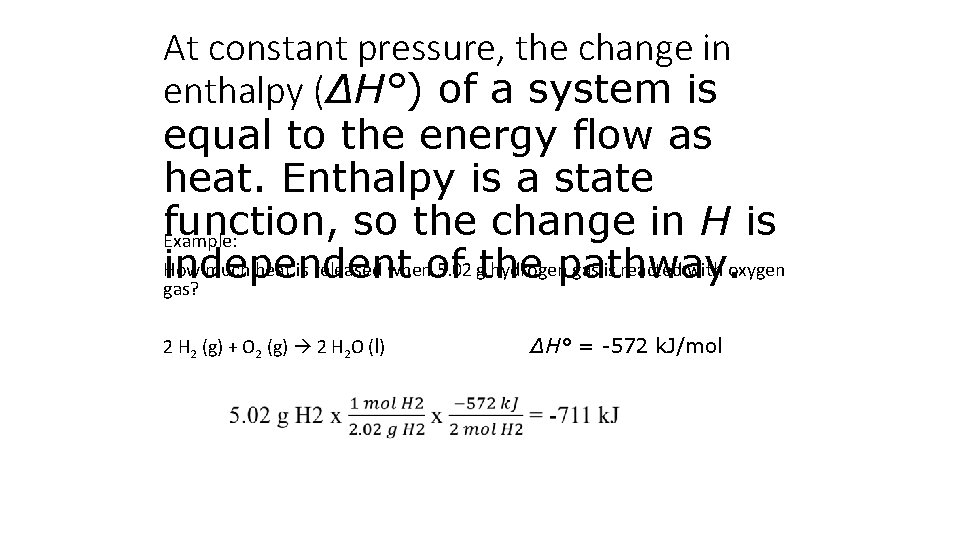
At constant pressure, the change in enthalpy (ΔH°) of a system is equal to the energy flow as heat. Enthalpy is a state function, so the change in H is Example: How much heat is released whenof 5. 02 gthe hydrogen gas is reacted with oxygen independent pathway. gas? 2 H 2 (g) + O 2 (g) 2 H 2 O (l) ΔH° = -572 k. J/mol
Exercise The overall reaction in a commercial heat pack can be represented as: 4 Fe (s) + 3 O 2 (g) 2 Fe 2 O 3 (s) ΔH = – 1652 k. J a. How much heat is released when 4. 00 moles of iron is reacted with excess oxygen gas? Copyright © Cengage Learning. All rights reserved 7

Exercise The overall reaction in a commercial heat pack can be represented as: 4 Fe (s) + 3 O 2 (g) 2 Fe 2 O 3 (s) ΔH = – 1652 k. J b. How much heat is released when 1. 00 moles of iron (III) oxide is produced? Copyright © Cengage Learning. All rights reserved 8

Exercise The overall reaction in a commercial heat pack can be represented as: 4 Fe (s) + 3 O 2 (g) 2 Fe 2 O 3 (s) ΔH = – 1652 k. J c. How many moles of iron are reacted in excess oxygen to produce 533 kilojoules of energy? Copyright © Cengage Learning. All rights reserved 9
Exercise The overall reaction in a commercial heat pack can be represented as: 4 Fe (s) + 3 O 2 (g) 2 Fe 2 O 3 (s) ΔH = – 1652 k. J d. How much heat is released when 1. 00 g of iron is reacted with excess oxygen gas? Copyright © Cengage Learning. All rights reserved 10

Exercise The overall reaction in a commercial heat pack can be represented as: 4 Fe (s) + 3 O 2 (g) 2 Fe 2 O 3 (s) ΔH = – 1652 k. J e. How much heat is released when 10. 0 g Fe and 2. 00 g of O 2 are reacted? Copyright © Cengage Learning. All rights reserved 11

Exercise Consider the combustion of propane: C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(l) ΔH = – 2221 k. J Assume that all of the heat comes from the combustion of propane. Calculate ΔH in which 5. 00 g of propane is burned in excess oxygen at constant pressure. – 252 k. J Copyright © Cengage Learning. All rights reserved 12



• The common energy units for heat are the calorie and the joule. § calorie – the amount of energy (heat) required to raise the temperature of one gram of water 1 o. C. § Joule – 1 calorie = 4. 184 joules Copyright © Cengage Learning. All rights reserved 15

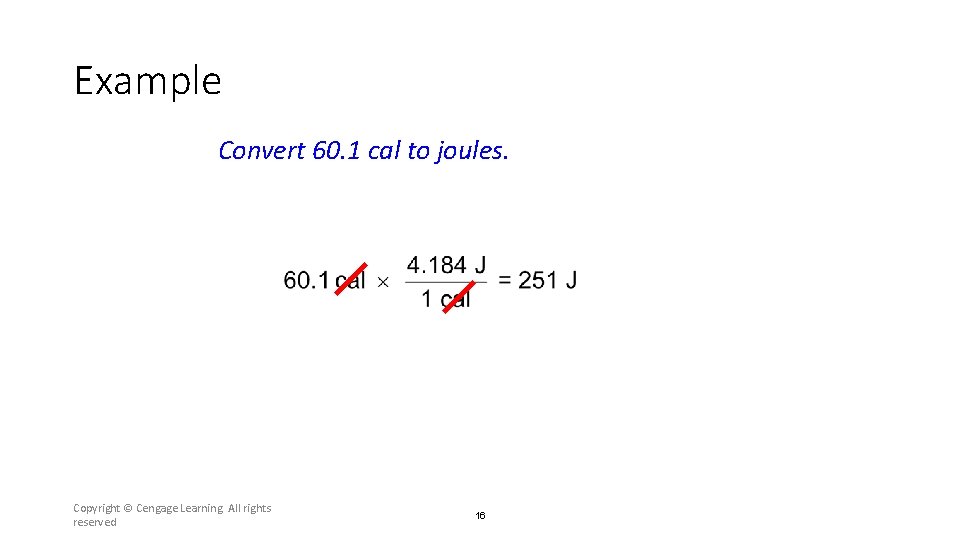
Example Convert 60. 1 cal to joules. Copyright © Cengage Learning. All rights reserved 16

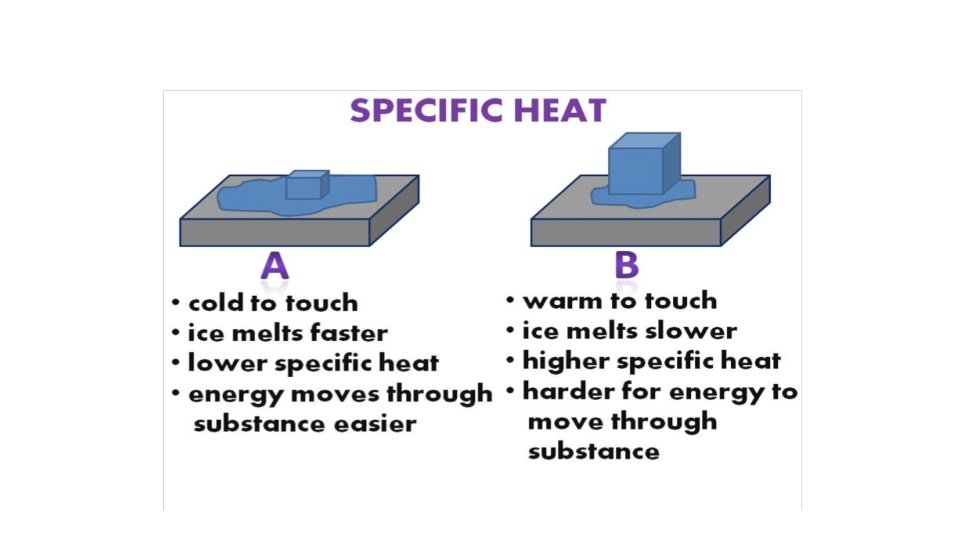
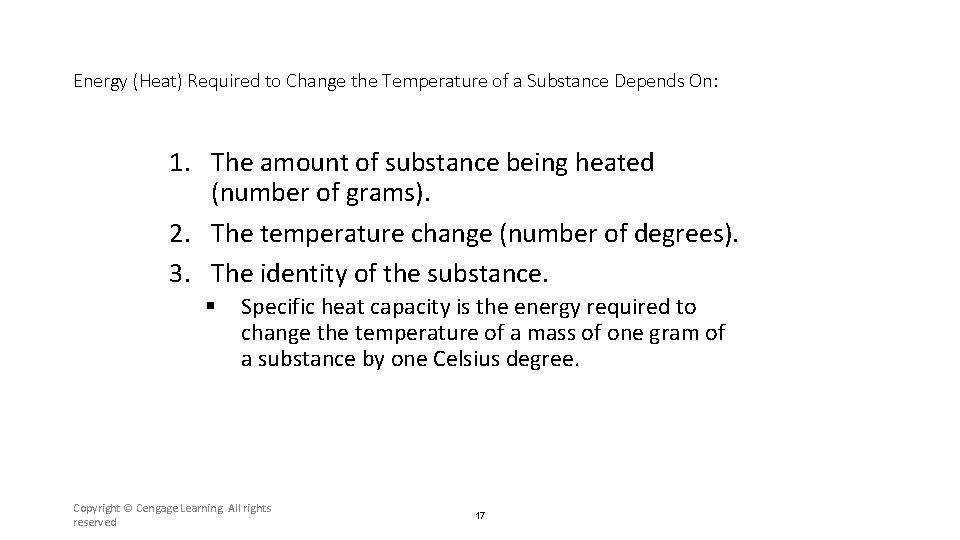
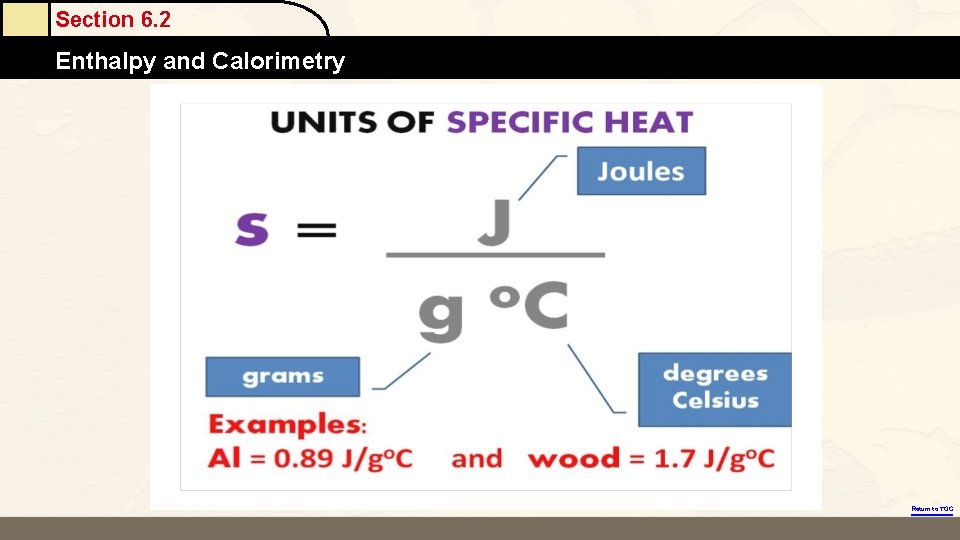
Energy (Heat) Required to Change the Temperature of a Substance Depends On: 1. The amount of substance being heated (number of grams). 2. The temperature change (number of degrees). 3. The identity of the substance. § Specific heat capacity is the energy required to change the temperature of a mass of one gram of a substance by one Celsius degree. Copyright © Cengage Learning. All rights reserved 17


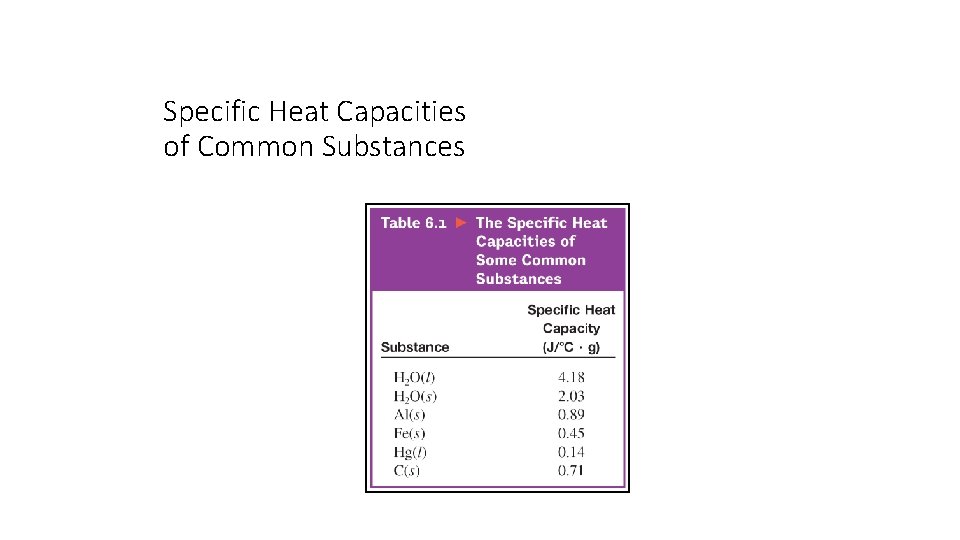
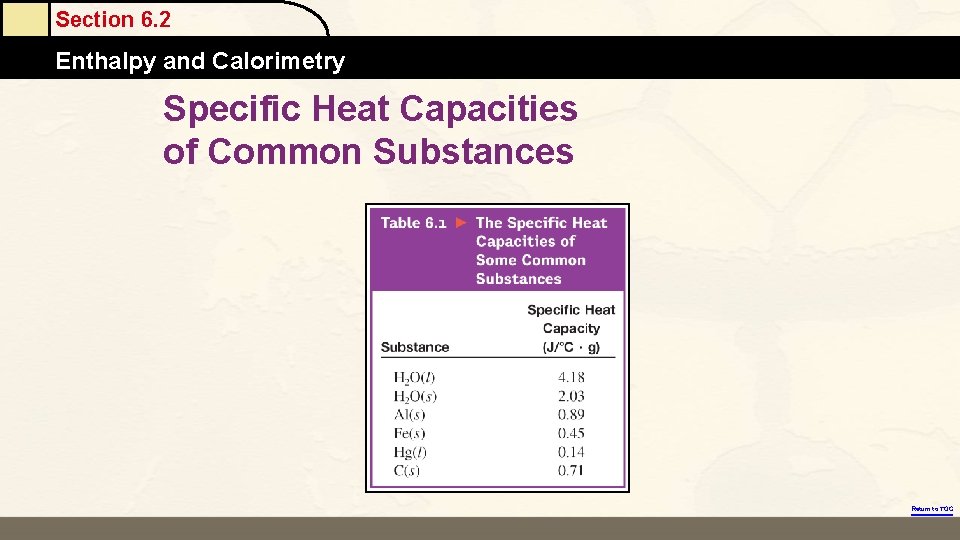
Specific Heat Capacities of Common Substances

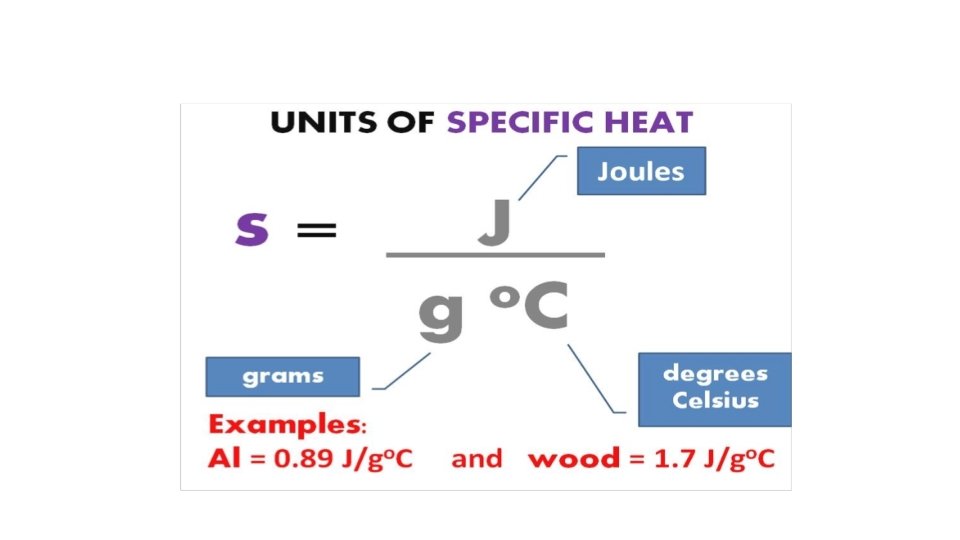
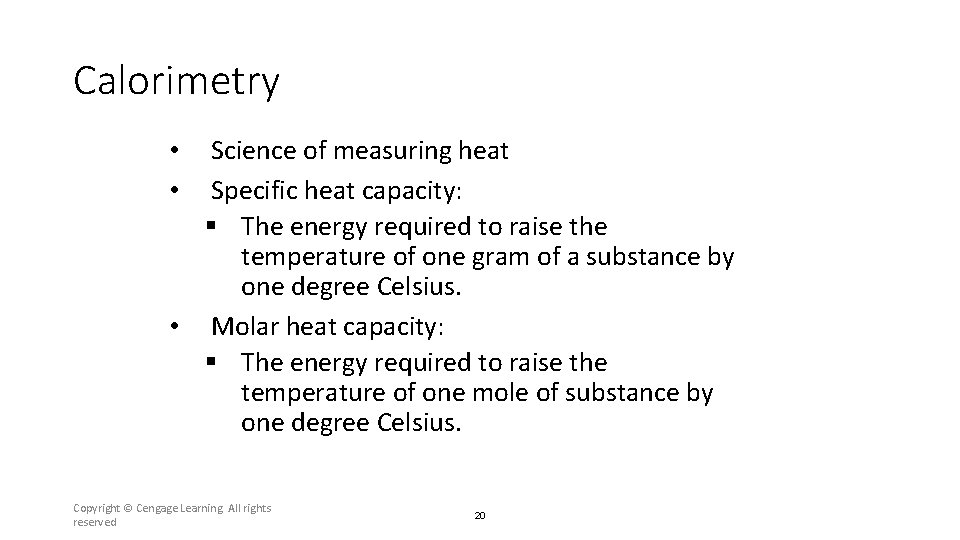
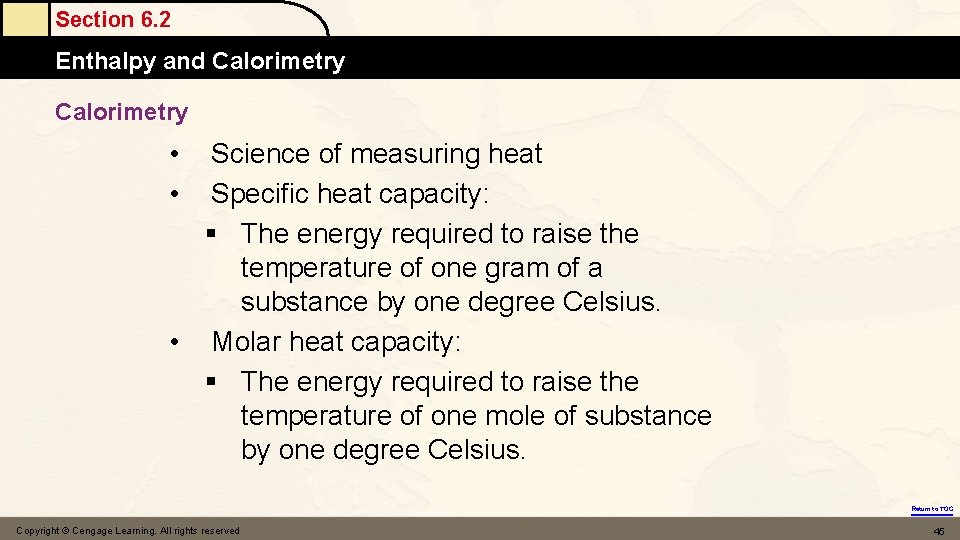
Calorimetry Science of measuring heat Specific heat capacity: § The energy required to raise the temperature of one gram of a substance by one degree Celsius. • Molar heat capacity: § The energy required to raise the temperature of one mole of substance by one degree Celsius. • • Copyright © Cengage Learning. All rights reserved 20

Q = smΔT

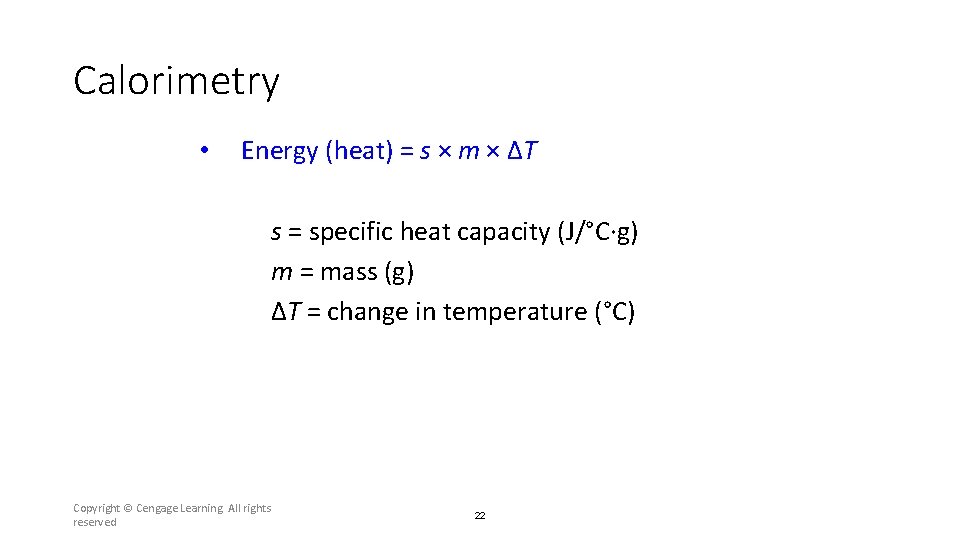
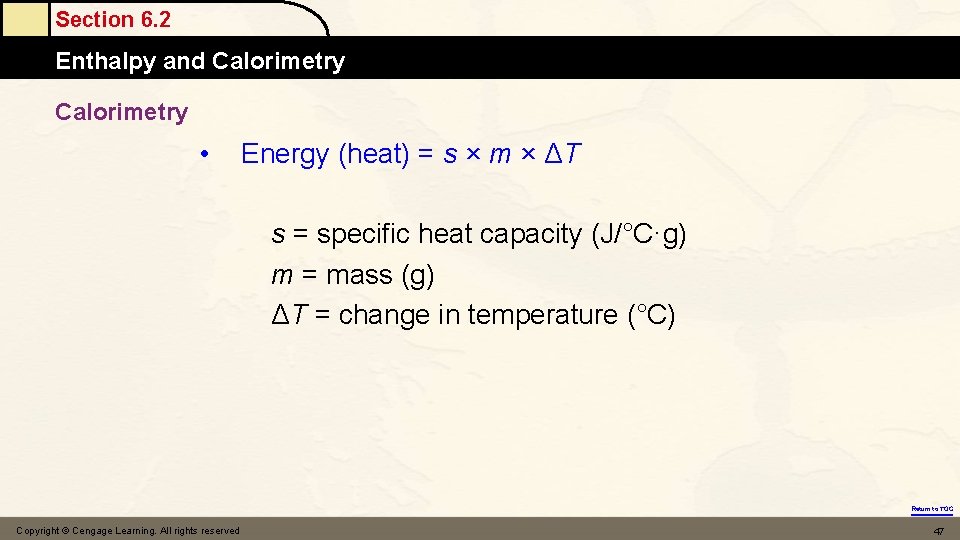
Calorimetry • Energy (heat) = s × m × ΔT s = specific heat capacity (J/°C·g) m = mass (g) ΔT = change in temperature (°C) Copyright © Cengage Learning. All rights reserved 22




= -7900 J -1. 9 kcal

Section 6. 1 The Nature of Energy Concept Check What is the change in internal energy of a system that gives off 89 J of heat and has 531 J of work done on it? q = -89 J w = +531 J ΔE = (-89 J) + (+531 J) = 442 J Return to TOC Copyright © Cengage Learning. All rights reserved 27

Section 6. 1 The Nature of Energy Concept Check Determine the sign of E for each of the following with the listed conditions: a) An endothermic process that performs work. § |work| > |heat| Δ E = negative § |work| < |heat| Δ E = positive b) Work is done on a gas and the process is exothermic. § |work| > |heat| Δ E = positive § |work| < |heat| Δ E = negative Return to TOC Copyright © Cengage Learning. All rights reserved 28

Section 6. 1 The Nature of Energy Homework: Read 10. 4 and 10. 5 and do self-checks Return to TOC

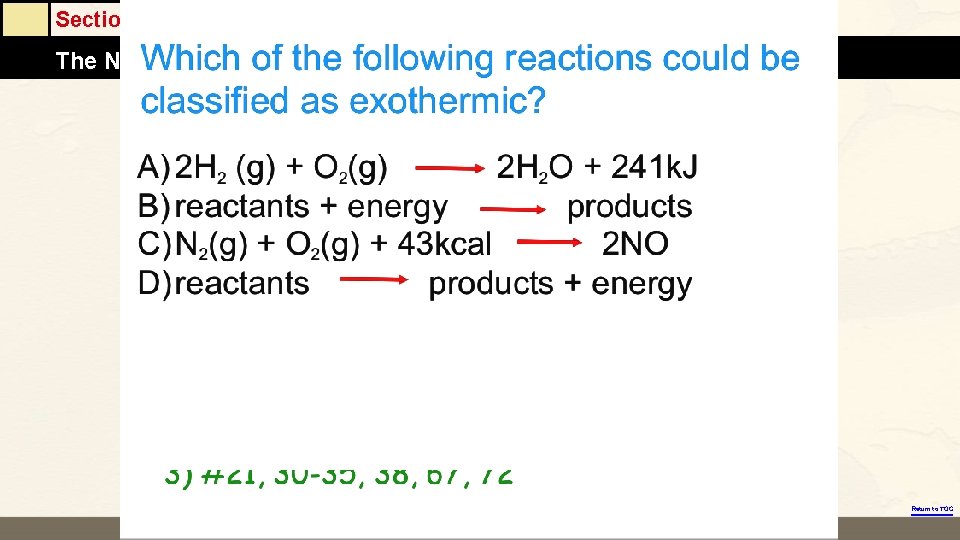
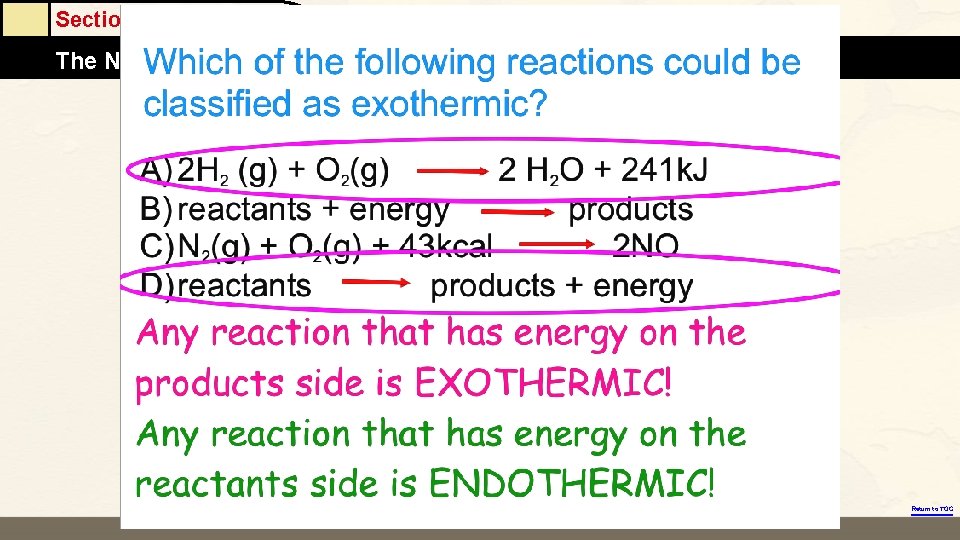
Section 6. 1 The Nature of Energy Return to TOC

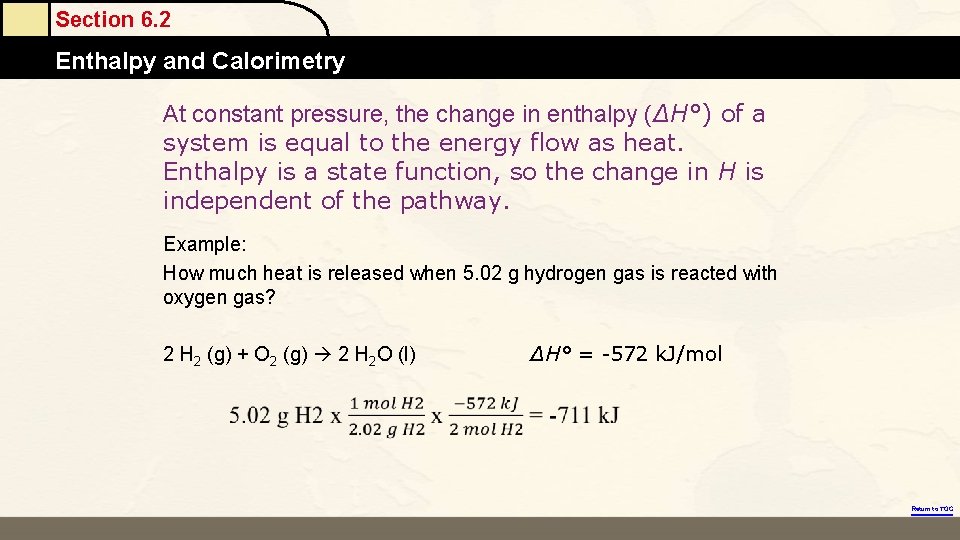
Section 6. 2 Enthalpy and Calorimetry At constant pressure, the change in enthalpy (ΔH°) of a system is equal to the energy flow as heat. Enthalpy is a state function, so the change in H is independent of the pathway. Example: How much heat is released when 5. 02 g hydrogen gas is reacted with oxygen gas? 2 H 2 (g) + O 2 (g) 2 H 2 O (l) ΔH° = -572 k. J/mol Return to TOC

Section 6. 2 Enthalpy and Calorimetry Exercise The overall reaction in a commercial heat pack can be represented as: 4 Fe (s) + 3 O 2 (g) 2 Fe 2 O 3 (s) ΔH = – 1652 k. J a. How much heat is released when 4. 00 moles of iron is reacted with excess oxygen gas? Return to TOC Copyright © Cengage Learning. All rights reserved 32

Section 6. 2 Enthalpy and Calorimetry Exercise The overall reaction in a commercial heat pack can be represented as: 4 Fe (s) + 3 O 2 (g) 2 Fe 2 O 3 (s) ΔH = – 1652 k. J b. How much heat is released when 1. 00 moles of iron (III) oxide is produced? Return to TOC Copyright © Cengage Learning. All rights reserved 33

Section 6. 2 Enthalpy and Calorimetry Exercise The overall reaction in a commercial heat pack can be represented as: 4 Fe (s) + 3 O 2 (g) 2 Fe 2 O 3 (s) ΔH = – 1652 k. J c. How many moles of iron are reacted in excess oxygen to produce 533 kilojoules of energy? Return to TOC Copyright © Cengage Learning. All rights reserved 34

Section 6. 2 Enthalpy and Calorimetry Exercise The overall reaction in a commercial heat pack can be represented as: 4 Fe (s) + 3 O 2 (g) 2 Fe 2 O 3 (s) ΔH = – 1652 k. J d. How much heat is released when 1. 00 g of iron is reacted with excess oxygen gas? Return to TOC Copyright © Cengage Learning. All rights reserved 35

Section 6. 2 Enthalpy and Calorimetry Exercise The overall reaction in a commercial heat pack can be represented as: 4 Fe (s) + 3 O 2 (g) 2 Fe 2 O 3 (s) ΔH = – 1652 k. J e. How much heat is released when 10. 0 g Fe and 2. 00 g of O 2 are reacted? Return to TOC Copyright © Cengage Learning. All rights reserved 36

Section 6. 2 Enthalpy and Calorimetry Exercise Consider the combustion of propane: C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(l) ΔH = – 2221 k. J Assume that all of the heat comes from the combustion of propane. Calculate ΔH in which 5. 00 g of propane is burned in excess oxygen at constant pressure. – 252 k. J Copyright © Cengage Learning. All rights reserved Return to TOC 37

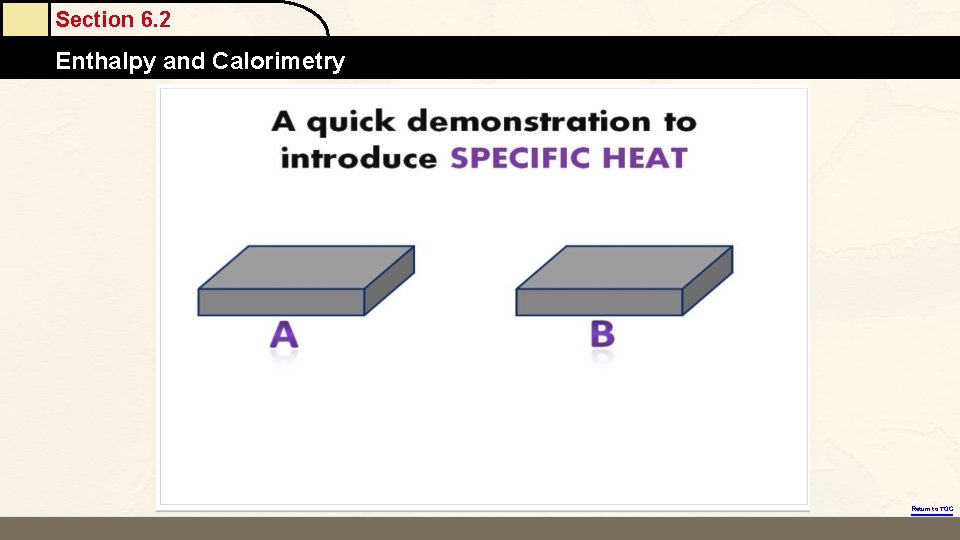
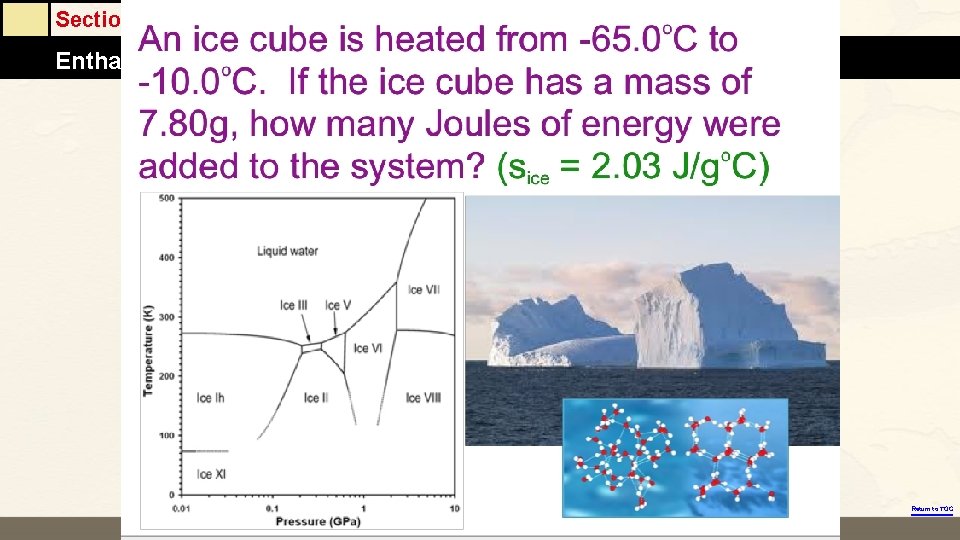
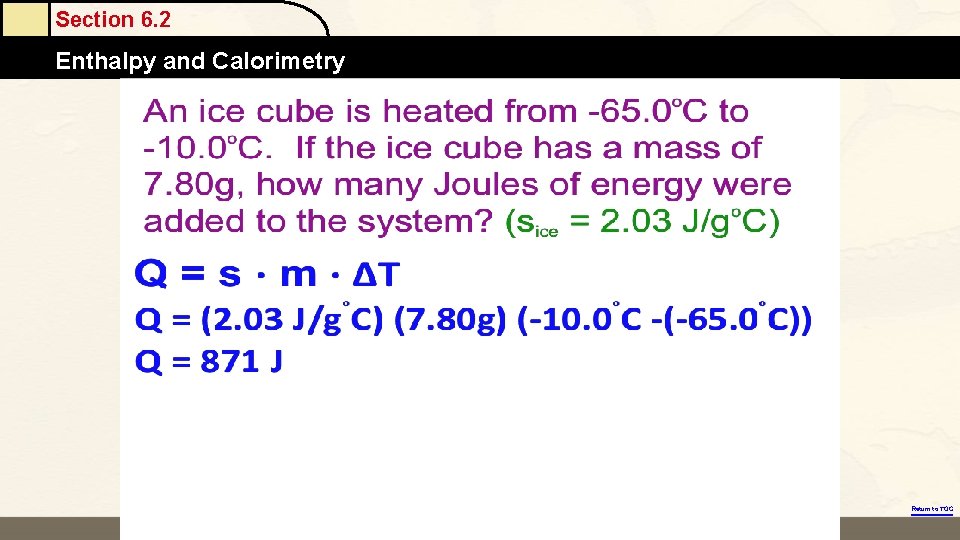
Section 6. 2 Enthalpy and Calorimetry Return to TOC

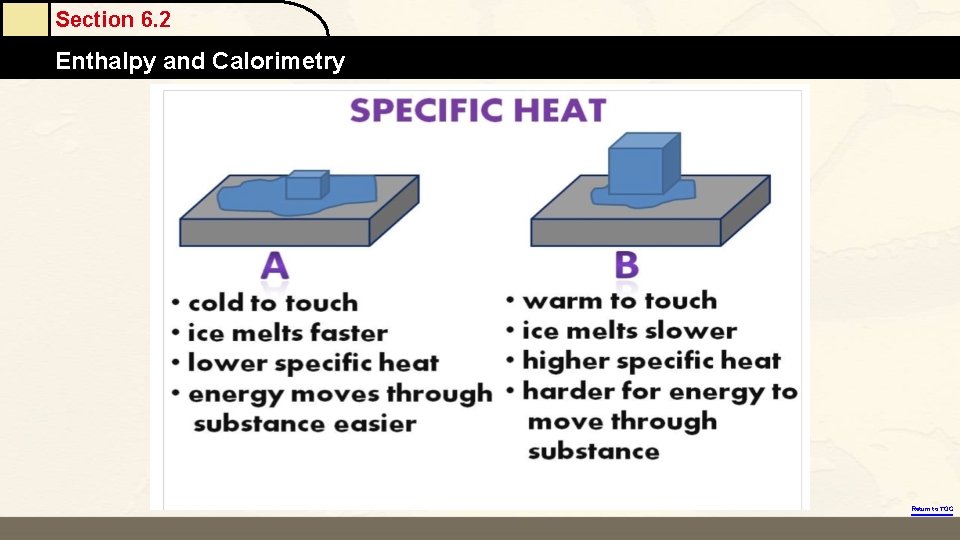
Section 6. 2 Enthalpy and Calorimetry Return to TOC

Section 6. 2 Enthalpy and Calorimetry • The common energy units for heat are the calorie and the joule. § calorie – the amount of energy (heat) required to raise the temperature of one gram of water 1 o. C. § Joule – 1 calorie = 4. 184 joules Return to TOC Copyright © Cengage Learning. All rights reserved 40

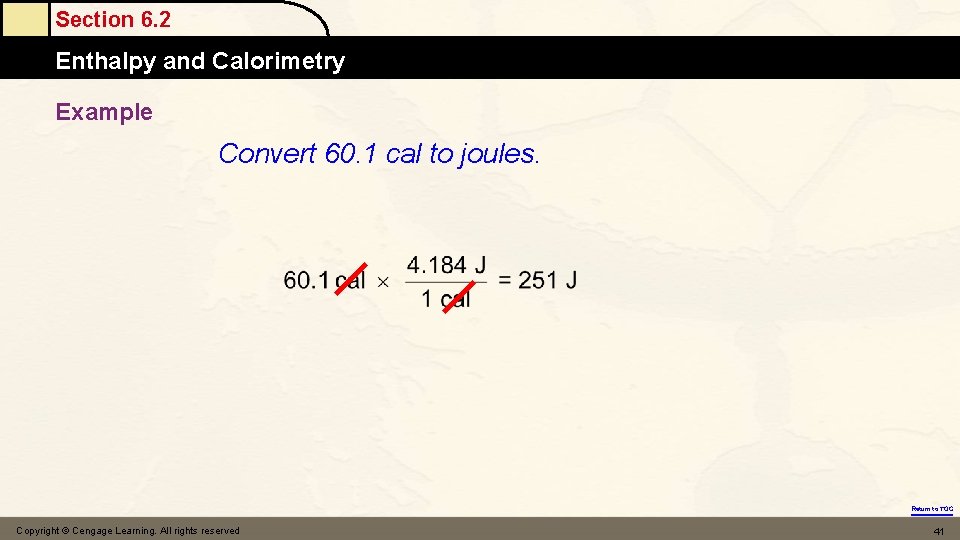
Section 6. 2 Enthalpy and Calorimetry Example Convert 60. 1 cal to joules. Return to TOC Copyright © Cengage Learning. All rights reserved 41

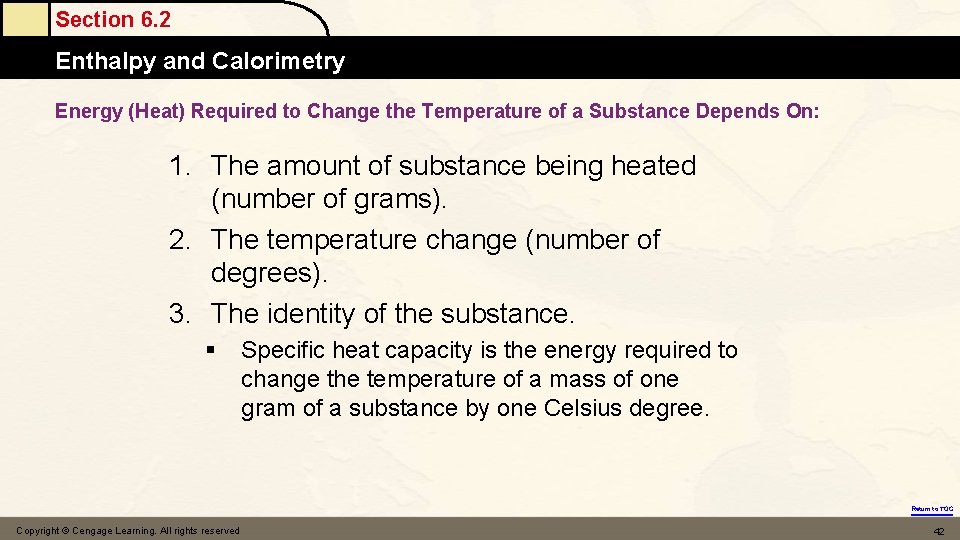
Section 6. 2 Enthalpy and Calorimetry Energy (Heat) Required to Change the Temperature of a Substance Depends On: 1. The amount of substance being heated (number of grams). 2. The temperature change (number of degrees). 3. The identity of the substance. § Specific heat capacity is the energy required to change the temperature of a mass of one gram of a substance by one Celsius degree. Return to TOC Copyright © Cengage Learning. All rights reserved 42

Section 6. 2 Enthalpy and Calorimetry Return to TOC

Section 6. 2 Enthalpy and Calorimetry Specific Heat Capacities of Common Substances Return to TOC

Section 6. 2 Enthalpy and Calorimetry • • Science of measuring heat Specific heat capacity: § The energy required to raise the temperature of one gram of a substance by one degree Celsius. • Molar heat capacity: § The energy required to raise the temperature of one mole of substance by one degree Celsius. Return to TOC Copyright © Cengage Learning. All rights reserved 45

Section 6. 2 Enthalpy and Calorimetry Q = smΔT Return to TOC

Section 6. 2 Enthalpy and Calorimetry • Energy (heat) = s × m × ΔT s = specific heat capacity (J/°C·g) m = mass (g) ΔT = change in temperature (°C) Return to TOC Copyright © Cengage Learning. All rights reserved 47

Section 6. 2 Enthalpy and Calorimetry Return to TOC

Section 6. 2 Enthalpy and Calorimetry Return to TOC

Section 6. 2 Enthalpy and Calorimetry Return to TOC

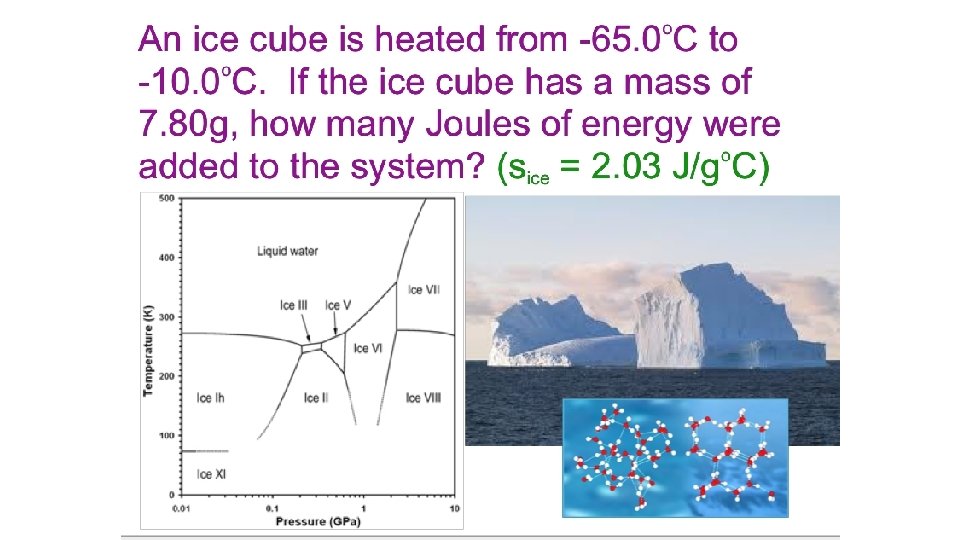
Section 6. 2 Enthalpy and Calorimetry = -7900 J -1. 9 kcal Return to TOC