Concentration of a Solution What is a Solution







- Slides: 10

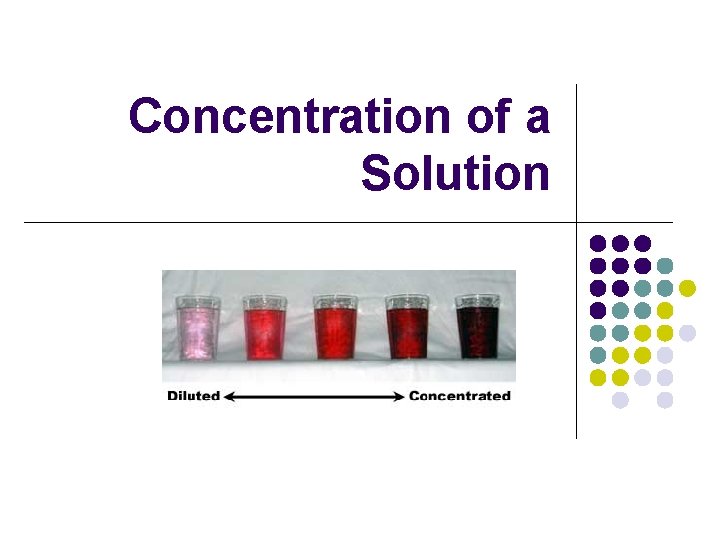
Concentration of a Solution

What is a Solution? l l A solution is a homogeneous mixture A homogeneous mixture is formed by one or more solutes and a single solvent l l Solute: the substance that is dissolved Solvent: the substance that does the dissolving In a solution, you cannot SEE the difference between the solute and solvent particles A solution looks as though it is only made of 1 substance, although it is really made up of 2 or more substances.

Example of a solution? l Kool- Aid!!! How do you make a glass of Kool-Aid? 1) 2) 3) Measure a small amount of Kool-Aid powder into a glass Add cold water to the glass and stir Enjoy! What is the solute and solvent in Kool-Aid? Solute: Kool-Aid powder Solvent: water

What is concentration? The concentration of a solution is the ratio of the amount of solute to the total quantity of solution Concentration = quantity of solute quantity of solution (not solvent)

Concentrated solution l l A concentrated solution contains large amounts of solute in comparison to the volume of the solution So let’s say you combine 4 spoonfuls of Kool. Aid and 1 cup of water in a glass, your solution would look very dark and taste very sweet. l this is a concentrated solution

Dilute solution l A dilute solution contains small amounts of solute in relation to the volume of a solution. l If you combine only 1 spoonful of Kool-Aid to the same volume of water (1 cup), your solution would appear lighter and would not taste as sweet l This is a dilute solution

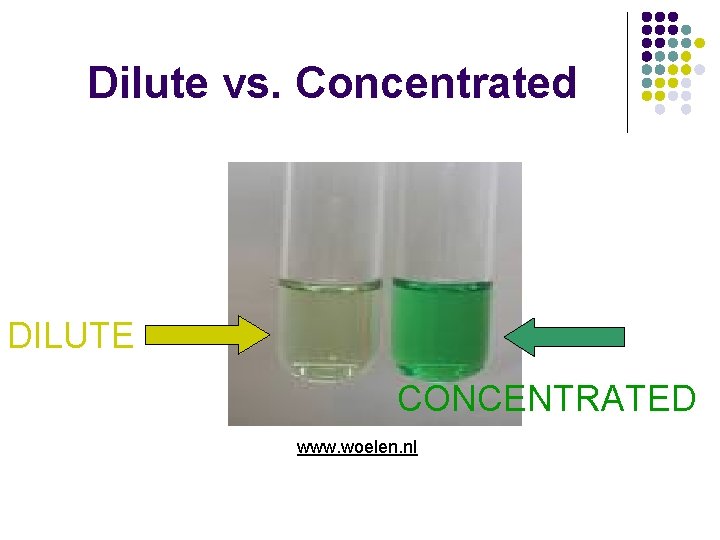
Dilute vs. Concentrated DILUTE CONCENTRATED www. woelen. nl

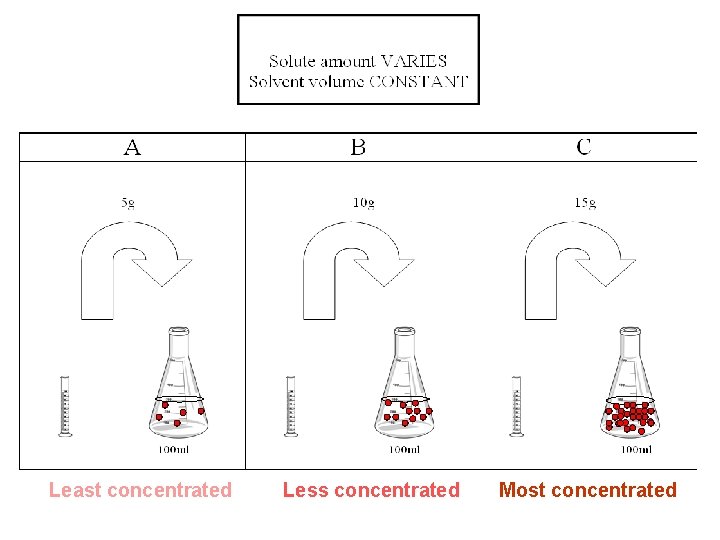
Most concentrated Less concentrated Least concentrated

Solute varies, solvent costant Least concentrated Less concentrated Most concentrated

Concentration Video