Concentrated Polymer Solutions 10302020 1 Application and Importance














- Slides: 116

Concentrated Polymer Solutions 10/30/2020 1

Application and Importance of Concentrated Polymer Solutions 10/30/2020 2

What does mean? • • A dilute solution Theta condition Semi-dilute Concentrated and so On. . 10/30/2020 3

What we expect from a concentrated solution? 10/30/2020 The phase separation …… 4

What are the requirements for phase separation. • The required conditions in terms of thermodynamical properties of the system. • The effect of temperature. • The effect of molecular weight. • The effect of concentration. 10/30/2020 5

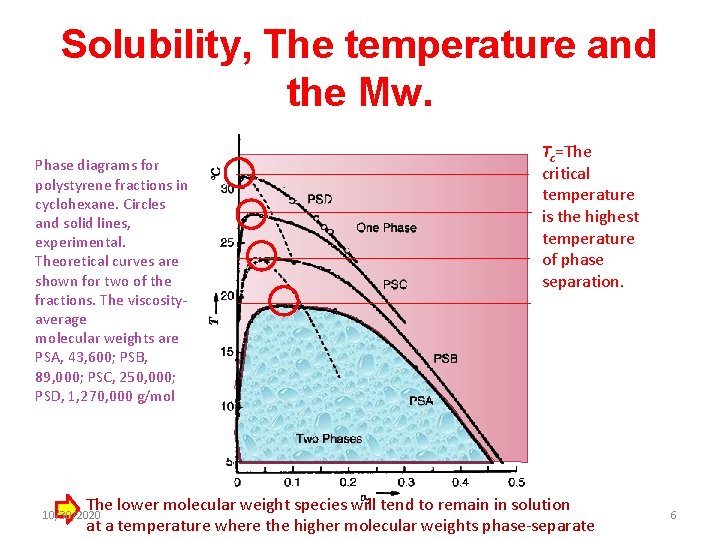
Solubility, The temperature and the Mw. Phase diagrams for polystyrene fractions in cyclohexane. Circles and solid lines, experimental. Theoretical curves are shown for two of the fractions. The viscosityaverage molecular weights are PSA, 43, 600; PSB, 89, 000; PSC, 250, 000; PSD, 1, 270, 000 g/mol Tc=The critical temperature is the highest temperature of phase separation. The lower molecular weight species will tend to remain in solution at a temperature where the higher molecular weights phase-separate 10/30/2020 6

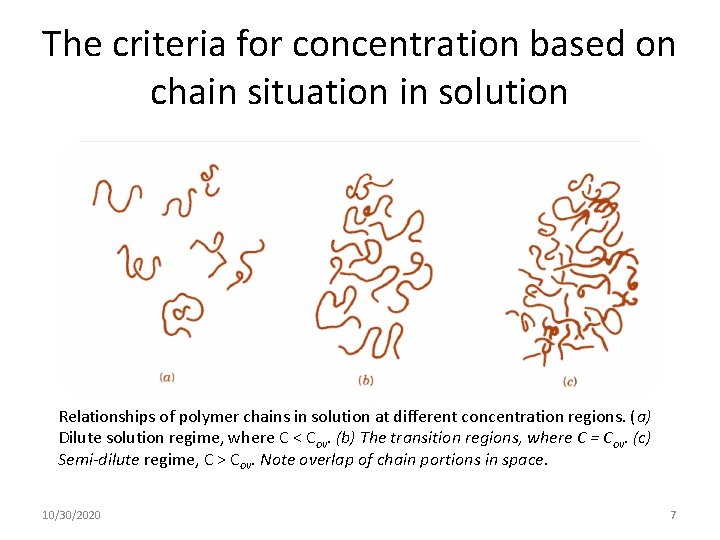
The criteria for concentration based on chain situation in solution Relationships of polymer chains in solution at different concentration regions. (a) Dilute solution regime, where C < Cov. (b) The transition regions, where C = Cov. (c) Semi-dilute regime, C > Cov. Note overlap of chain portions in space. 10/30/2020 7

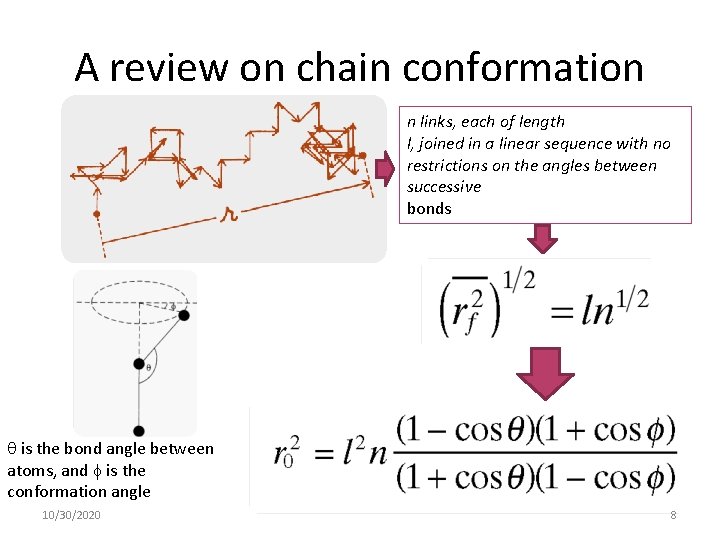
A review on chain conformation n links, each of length l, joined in a linear sequence with no restrictions on the angles between successive bonds is the bond angle between atoms, and is the conformation angle 10/30/2020 8

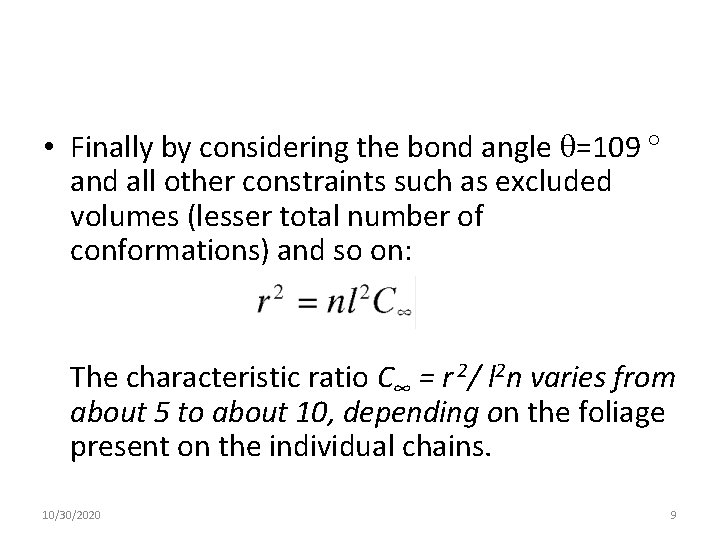
• Finally by considering the bond angle =109 and all other constraints such as excluded volumes (lesser total number of conformations) and so on: The characteristic ratio C∞ = r 2/ l 2 n varies from about 5 to about 10, depending on the foliage present on the individual chains. 10/30/2020 9

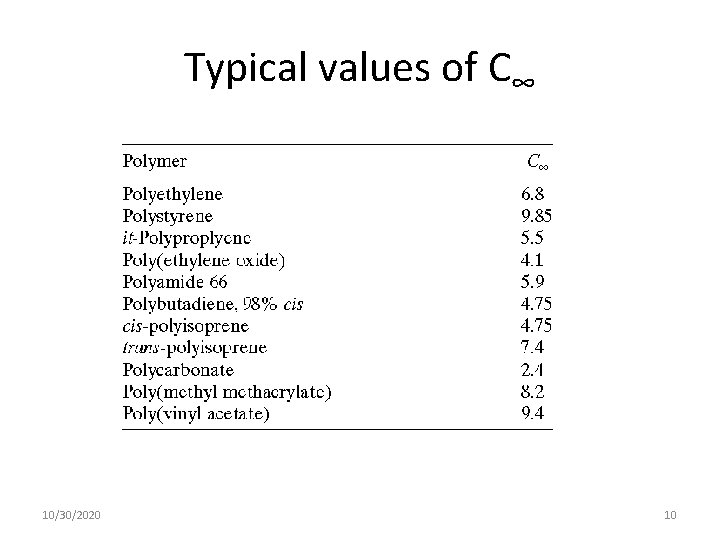
Typical values of C∞ 10/30/2020 10

Segmental lengths in Chains • Kuhn Segment Length 10/30/2020 11

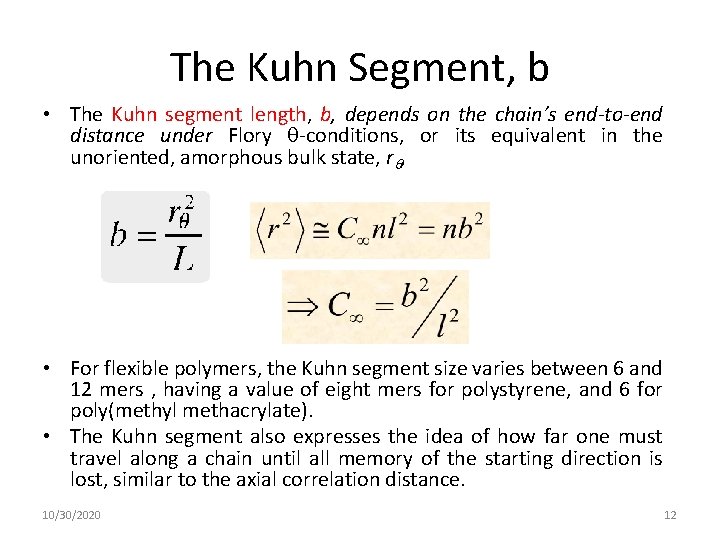
The Kuhn Segment, b • The Kuhn segment length, b, depends on the chain’s end-to-end distance under Flory -conditions, or its equivalent in the unoriented, amorphous bulk state, r , • For flexible polymers, the Kuhn segment size varies between 6 and 12 mers , having a value of eight mers for polystyrene, and 6 for poly(methyl methacrylate). • The Kuhn segment also expresses the idea of how far one must travel along a chain until all memory of the starting direction is lost, similar to the axial correlation distance. 10/30/2020 12

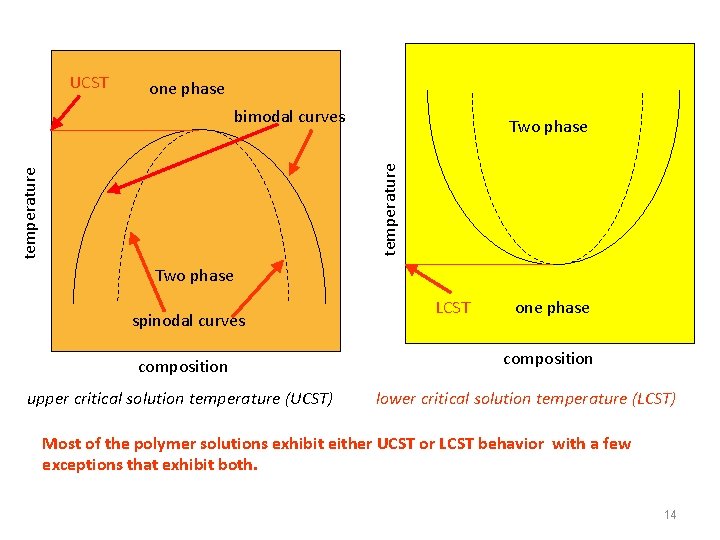
UCST one phase bimodal curves temperature Two phase spinodal curves composition upper critical solution temperature (UCST) LCST one phase composition lower critical solution temperature (LCST) Most of the polymer solutions exhibit either UCST or LCST behavior with a few exceptions that exhibit both. 14

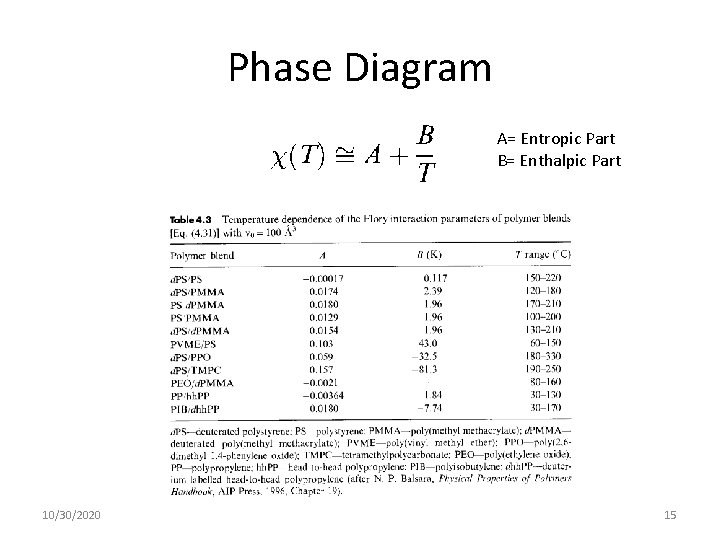
Phase Diagram A= Entropic Part B= Enthalpic Part 10/30/2020 15

Phase diagram At Theta Condition 10/30/2020 16

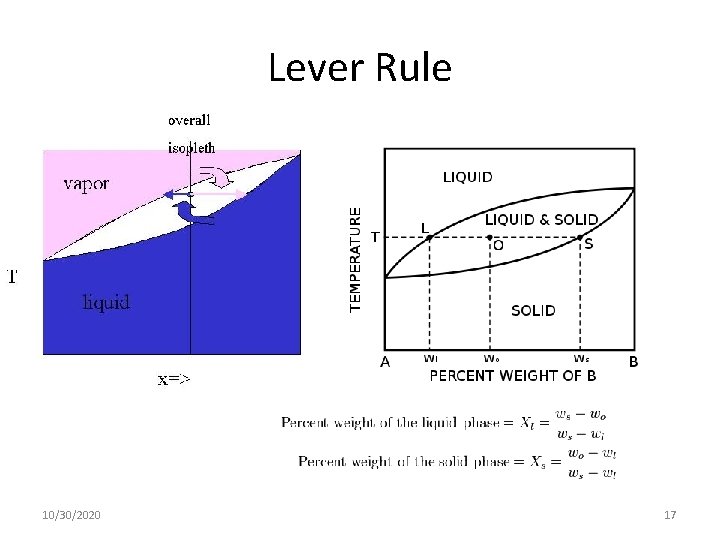
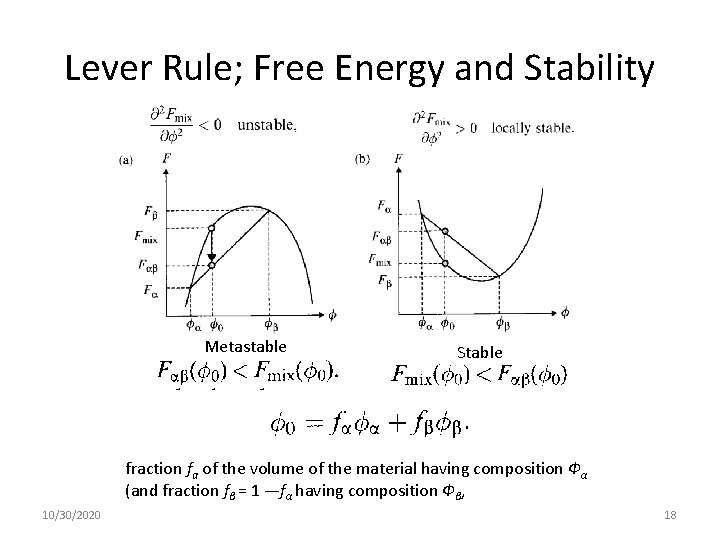
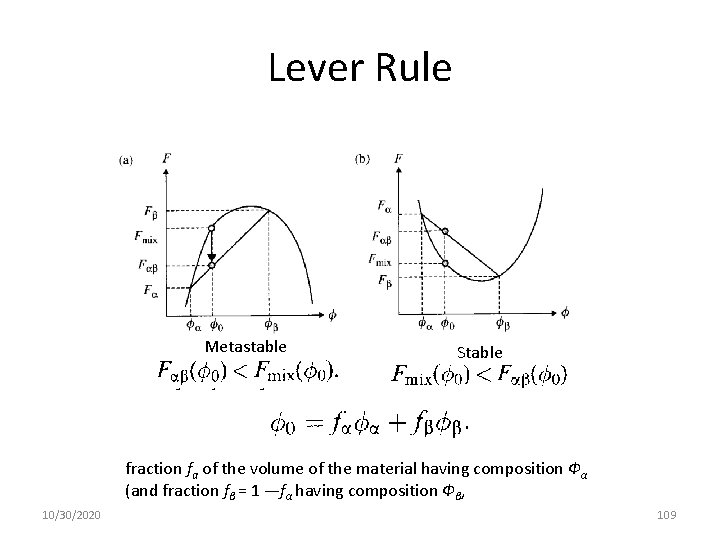
Lever Rule 10/30/2020 17

Lever Rule; Free Energy and Stability Metastable Stable fraction fa of the volume of the material having composition Фα (and fraction fβ = 1 —fα having composition Фβ, 10/30/2020 18

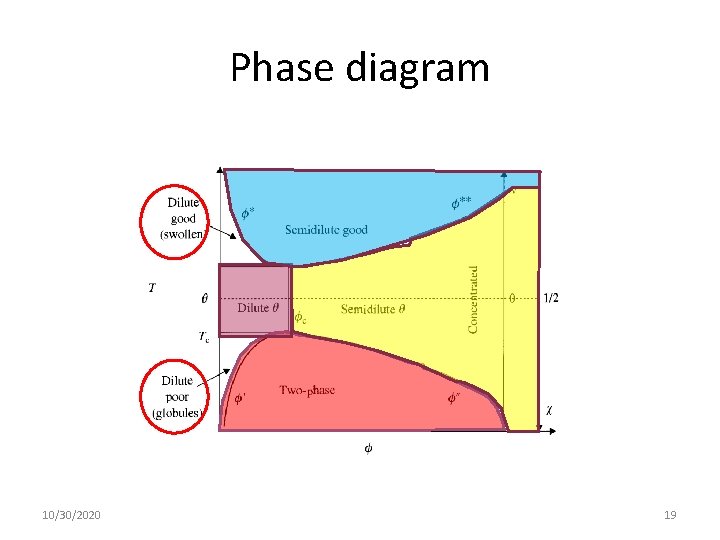
Phase diagram 10/30/2020 19

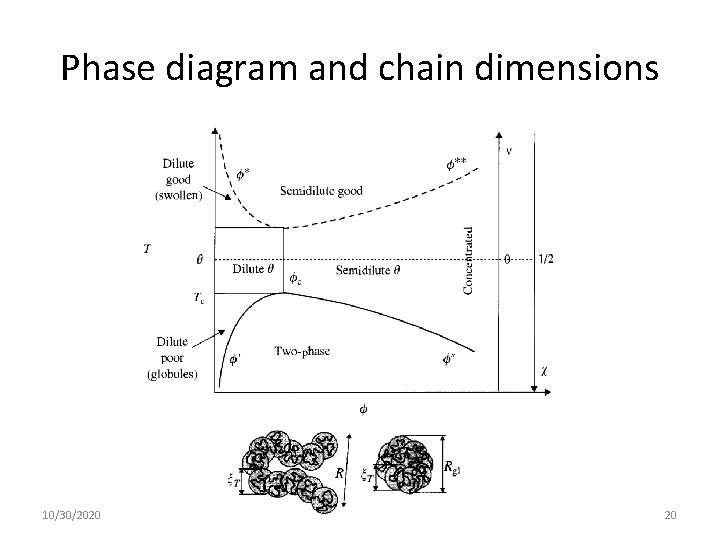
Phase diagram and chain dimensions 10/30/2020 20

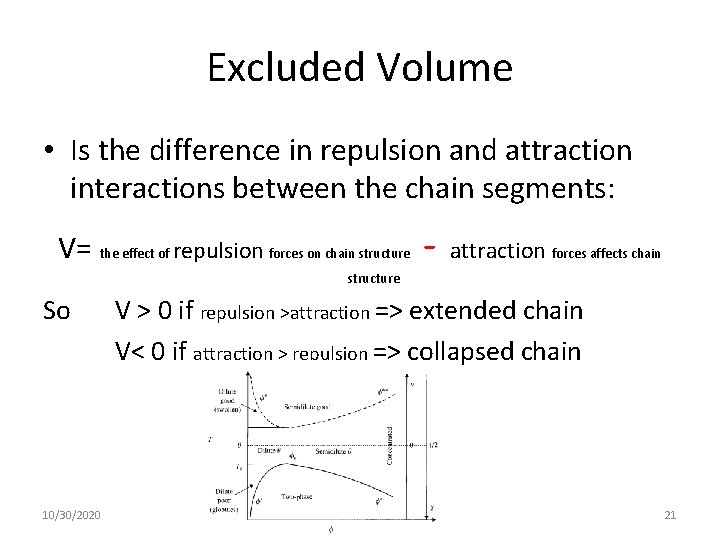
Excluded Volume • Is the difference in repulsion and attraction interactions between the chain segments: V= the effect of repulsion forces on chain structure So 10/30/2020 - attraction forces affects chain V > 0 if repulsion >attraction => extended chain V< 0 if attraction > repulsion => collapsed chain 21

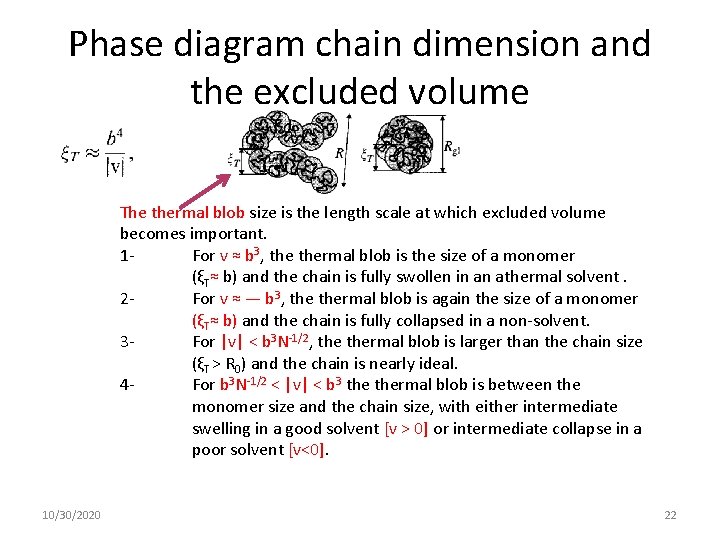
Phase diagram chain dimension and the excluded volume The thermal blob size is the length scale at which excluded volume becomes important. 1 For v ≈ b 3, thermal blob is the size of a monomer (ξT≈ b) and the chain is fully swollen in an athermal solvent. 2 For v ≈ — b 3, thermal blob is again the size of a monomer (ξT≈ b) and the chain is fully collapsed in a non-solvent. 3 For |v| < b 3 N-1/2, thermal blob is larger than the chain size (ξT > R 0) and the chain is nearly ideal. 4 For b 3 N-1/2 < |v| < b 3 thermal blob is between the monomer size and the chain size, with either intermediate swelling in a good solvent [v > 0] or intermediate collapse in a poor solvent [v<0]. 10/30/2020 22

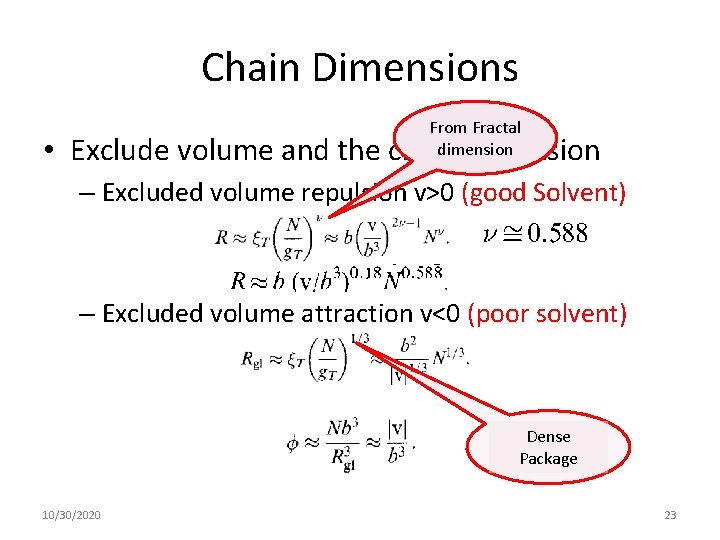
Chain Dimensions From Fractal dimension • Exclude volume and the chain dimension – Excluded volume repulsion v>0 (good Solvent) – Excluded volume attraction v<0 (poor solvent) Dense Package 10/30/2020 23

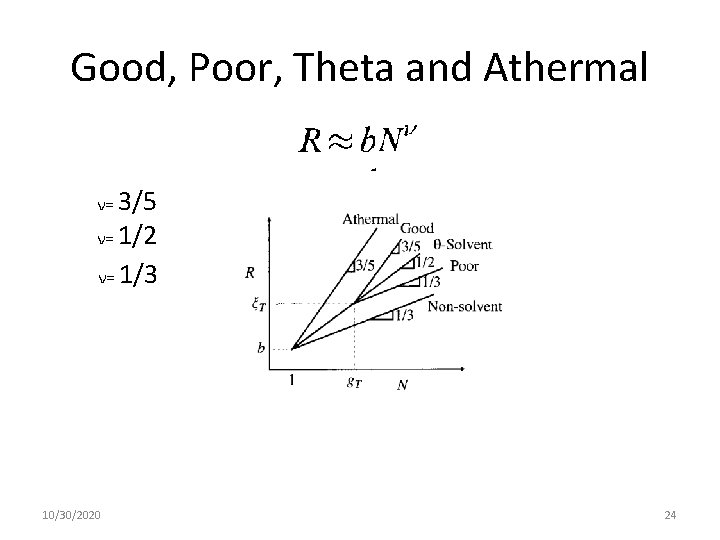
Good, Poor, Theta and Athermal ν= 3/5 ν= 1/2 ν= 1/3 10/30/2020 24

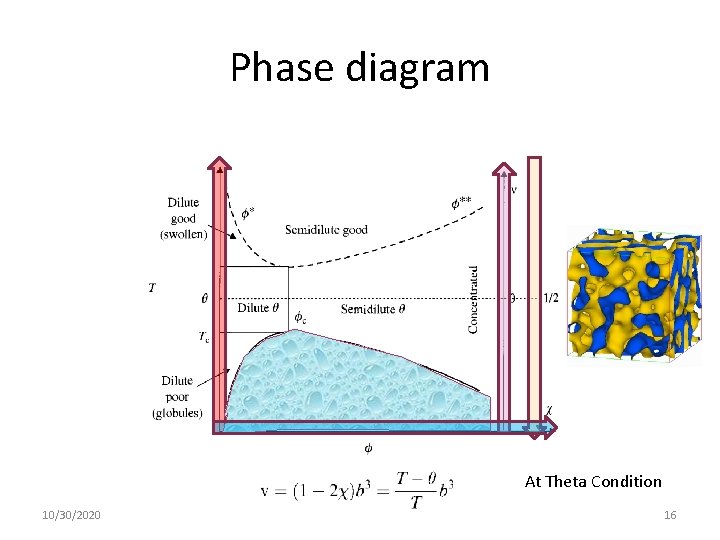
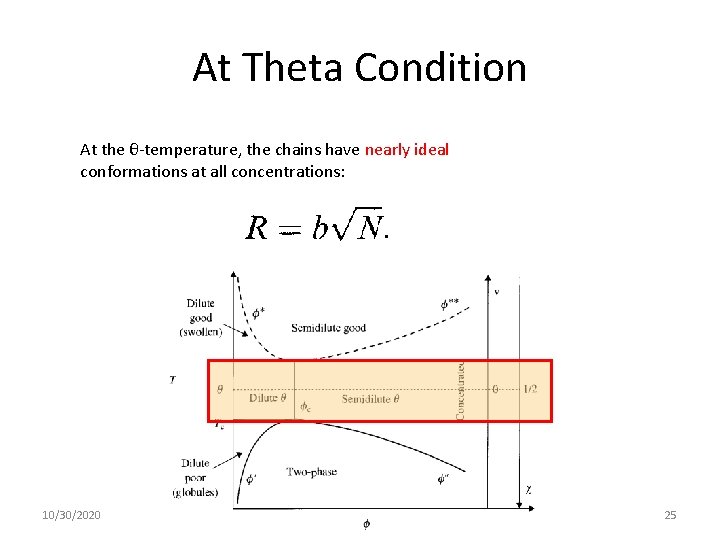
At Theta Condition At the θ-temperature, the chains have nearly ideal conformations at all concentrations: 10/30/2020 25

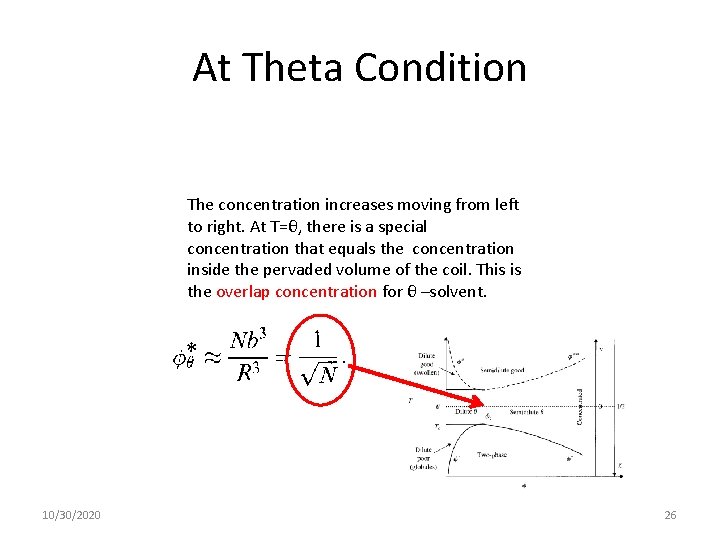
At Theta Condition The concentration increases moving from left to right. At T=θ, there is a special concentration that equals the concentration inside the pervaded volume of the coil. This is the overlap concentration for θ –solvent. 10/30/2020 26

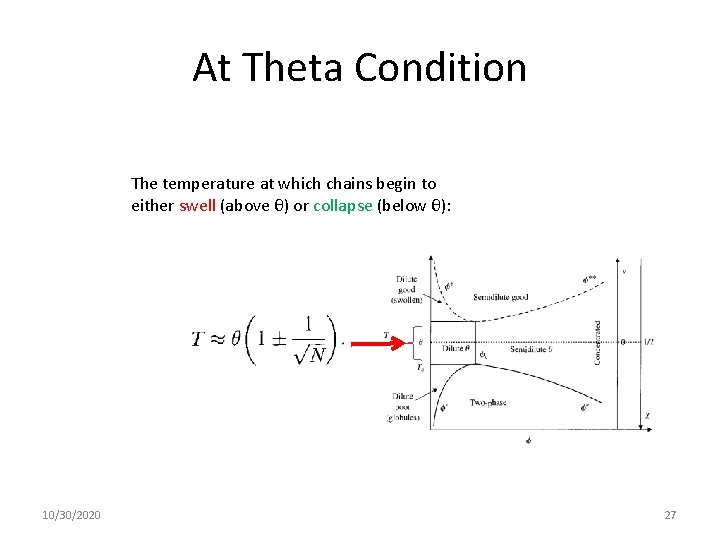
At Theta Condition The temperature at which chains begin to either swell (above θ) or collapse (below θ): 10/30/2020 27

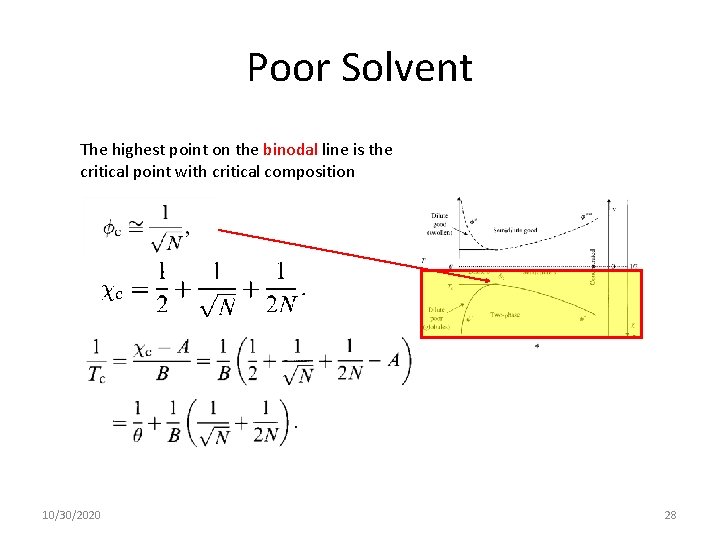
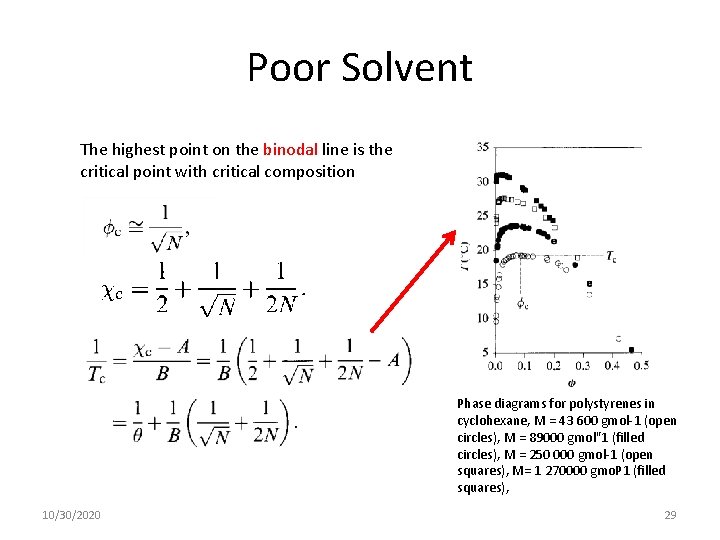
Poor Solvent The highest point on the binodal line is the critical point with critical composition 10/30/2020 28

Poor Solvent The highest point on the binodal line is the critical point with critical composition Phase diagrams for polystyrenes in cyclohexane, M = 43 600 gmol-1 (open circles), M = 89000 gmol"1 (filled circles), M = 250 000 gmol-1 (open squares), M= 1 270000 gmo. P 1 (filled squares), 10/30/2020 29

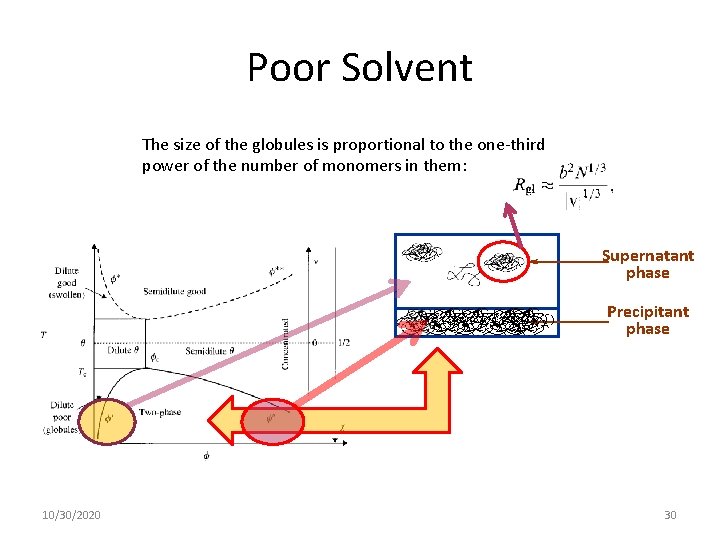
Poor Solvent The size of the globules is proportional to the one-third power of the number of monomers in them: Supernatant phase Precipitant phase 10/30/2020 30

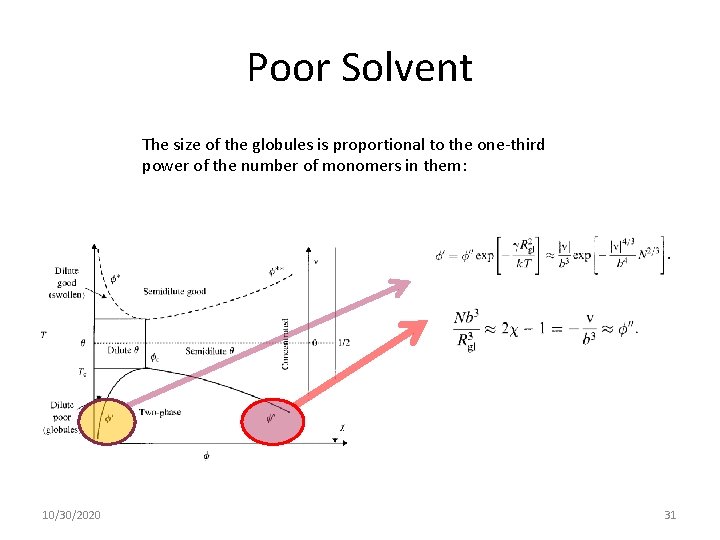
Poor Solvent The size of the globules is proportional to the one-third power of the number of monomers in them: 10/30/2020 31

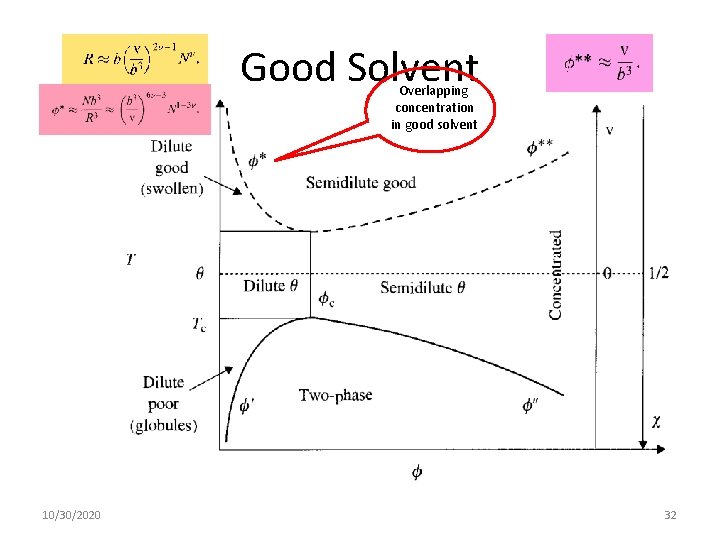
Good Solvent Overlapping concentration in good solvent 10/30/2020 32

What happens with the chain swelling in good solvents (due to the excluded volume) above the overlap concentration? • Important concept is that of the screening of excluded volume interactions in the concentrated solutions (Flory, Edwards): • as the chain concentration increases in the region , the coil swelling gradually diminishes and finally it vanishes in the melt (i. e. coils are ideal in the melt -Flory theorem). 10/30/2020 33

Polymer coil dimensions in semidilute solutions: example of scaling arguments 10/30/2020 34

Scaling Theory • Go for it…. 10/30/2020 35

Scaling Law And The Polymer Solvent Diagram 10/30/2020 40

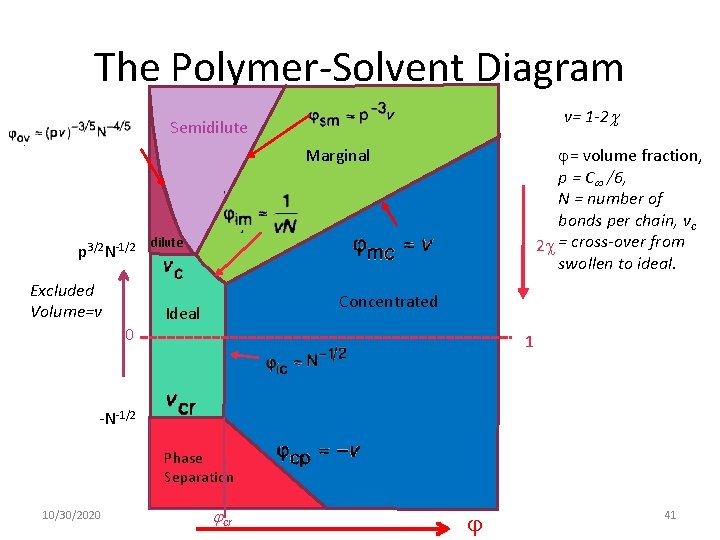
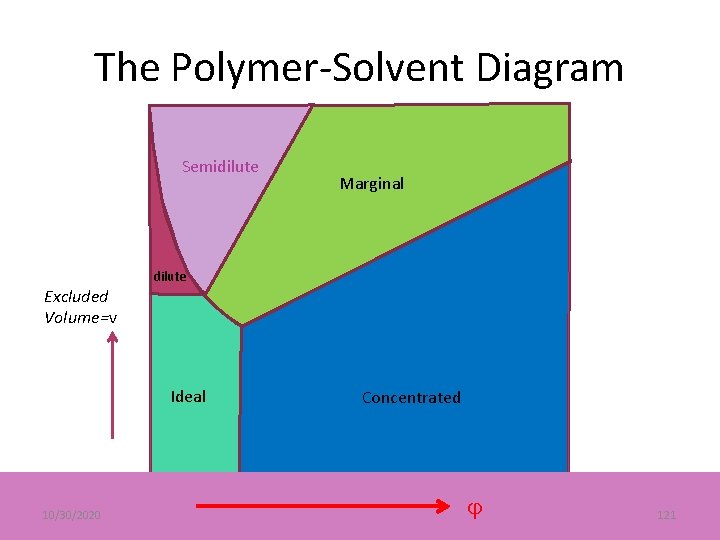
The Polymer-Solvent Diagram v= 1 -2 Semidilute = volume fraction, p = C /6, N = number of bonds per chain, vc 2 = cross-over from swollen to ideal. Marginal p 3/2 N-1/2 Excluded Volume=v 0 dilute Concentrated Ideal 1 -N-1/2 Phase Separation 10/30/2020 cr 41

Macroscopic Phase Separation 10/30/2020 42

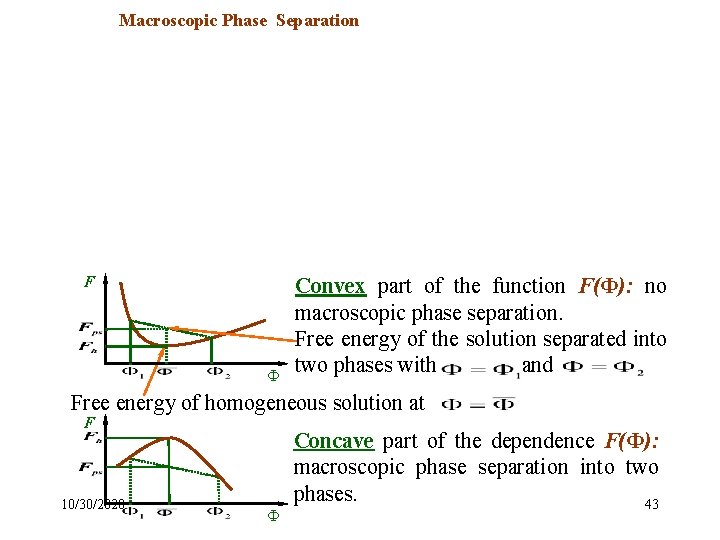
Macroscopic Phase Separation The typical dependence of the Flory-Huggins free energy on the polymer volume fraction in the solution : F 1 Spinodal points Ф Binodal points This dependence contains both convex and concave parts. F Ф Convex part of the function F(Ф): no macroscopic phase separation. Free energy of the solution separated into two phases with and Free energy of homogeneous solution at F 10/30/2020 Ф Concave part of the dependence F(Ф): macroscopic phase separation into two phases. 43

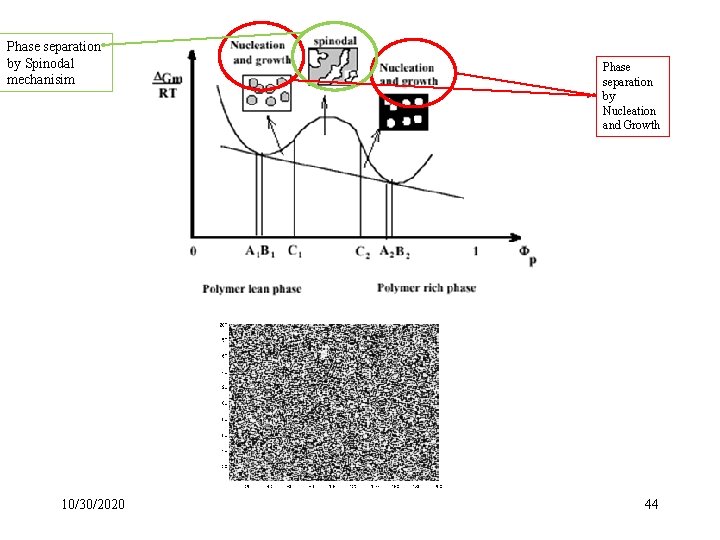
Phase separation by Spinodal mechanisim 10/30/2020 Phase separation by Nucleation and Growth 44

Some Review Slides 10/30/2020 45

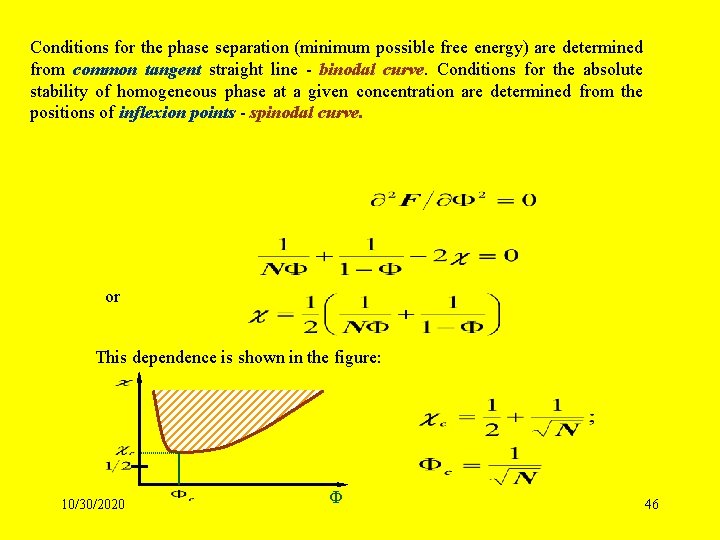
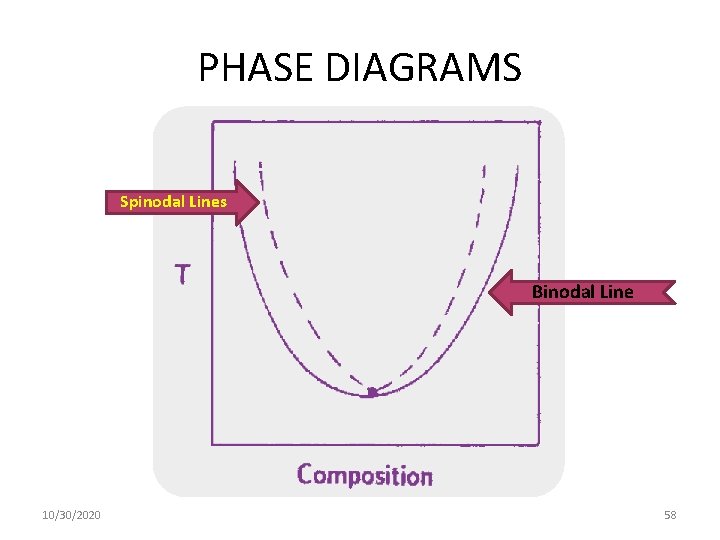
Conditions for the phase separation (minimum possible free energy) are determined from common tangent straight line - binodal curve. Conditions for the absolute stability of homogeneous phase at a given concentration are determined from the positions of inflexion points - spinodal curve. or This dependence is shown in the figure: 10/30/2020 Ф 46

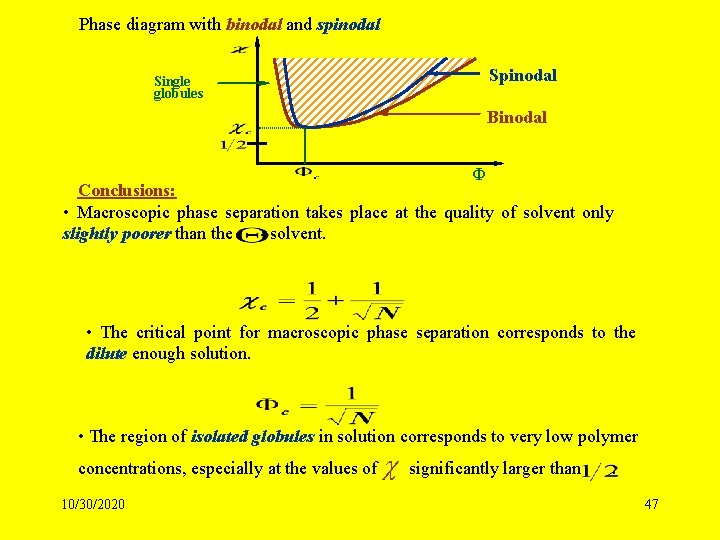
Phase diagram with binodal and spinodal Single globules Binodal Ф Conclusions: • Macroscopic phase separation takes place at the quality of solvent only slightly poorer than the - solvent. • The critical point for macroscopic phase separation corresponds to the dilute enough solution. • The region of isolated globules in solution corresponds to very low polymer concentrations, especially at the values of 10/30/2020 significantly larger than . 47

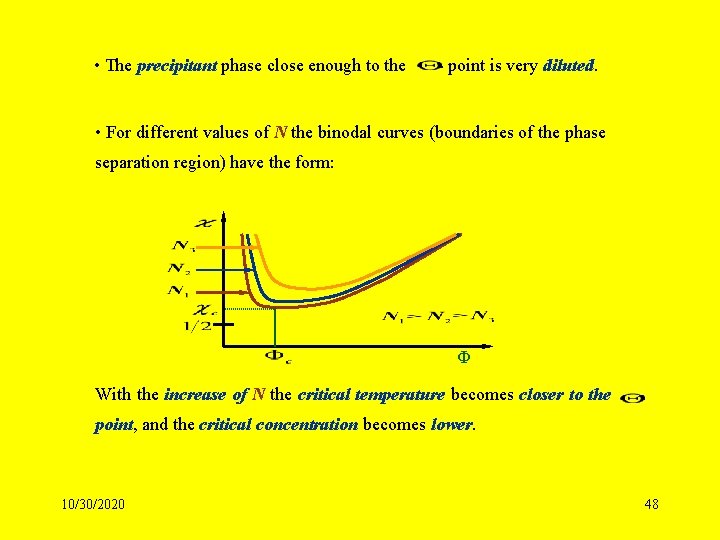
• The precipitant phase close enough to the - point is very diluted. • For different values of N the binodal curves (boundaries of the phase separation region) have the form: Ф With the increase of N the critical temperature becomes closer to the - point, and the critical concentration becomes lower. 10/30/2020 48

Method of fractional precipitation for polydisperse polymer solution: when the quality of solvent is becoming poorer or polymer concentration increases in the dilute enough range at first the most high-molecular fraction precipitates, then the next fraction, etc…; polymers with lower molecular weights require more significant increase in and to precipitate. In this way polymer fractionation is achieved. Reverse method is called the method of fractional dissolution: when one moves from the region of insolubility to the region of partial solubility at first the fractions with the lowest values of M are dissolved. 10/30/2020 49

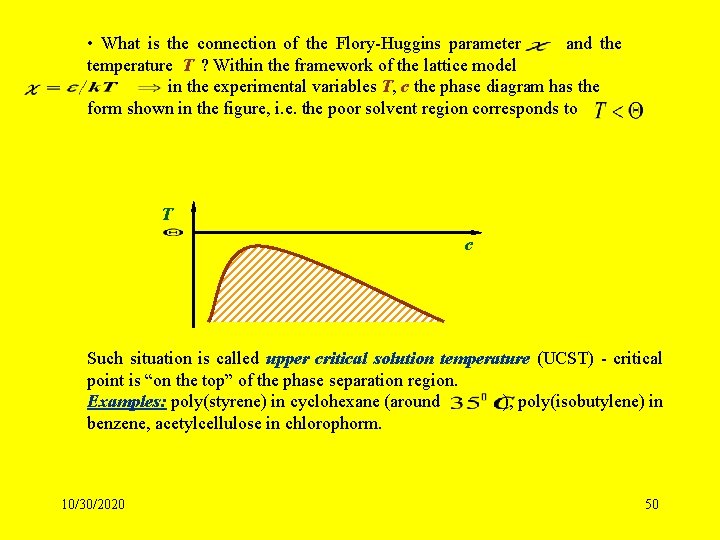
• What is the connection of the Flory-Huggins parameter and the temperature T ? Within the framework of the lattice model in the experimental variables T, c the phase diagram has the form shown in the figure, i. e. the poor solvent region corresponds to T c Such situation is called upper critical solution temperature (UCST) - critical point is “on the top” of the phase separation region. Examples: poly(styrene) in cyclohexane (around ), poly(isobutylene) in benzene, acetylcellulose in chlorophorm. 10/30/2020 50

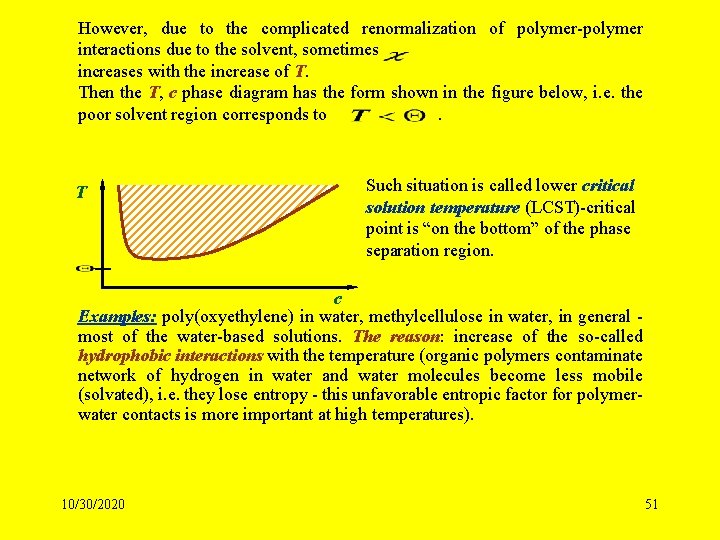
However, due to the complicated renormalization of polymer-polymer interactions due to the solvent, sometimes increases with the increase of T. Then the T, c phase diagram has the form shown in the figure below, i. e. the poor solvent region corresponds to. T Such situation is called lower critical solution temperature (LCST)-critical point is “on the bottom” of the phase separation region. c Examples: poly(oxyethylene) in water, methylcellulose in water, in general most of the water-based solutions. The reason: increase of the so-called hydrophobic interactions with the temperature (organic polymers contaminate network of hydrogen in water and water molecules become less mobile (solvated), i. e. they lose entropy - this unfavorable entropic factor for polymerwater contacts is more important at high temperatures). 10/30/2020 51

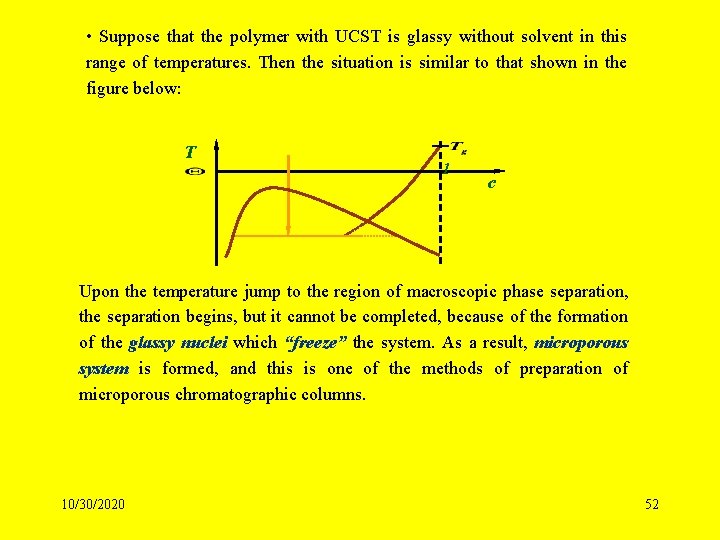
• Suppose that the polymer with UCST is glassy without solvent in this range of temperatures. Then the situation is similar to that shown in the figure below: T 1 c Upon the temperature jump to the region of macroscopic phase separation, the separation begins, but it cannot be completed, because of the formation of the glassy nuclei which “freeze” the system. As a result, microporous system is formed, and this is one of the methods of preparation of microporous chromatographic columns. 10/30/2020 52

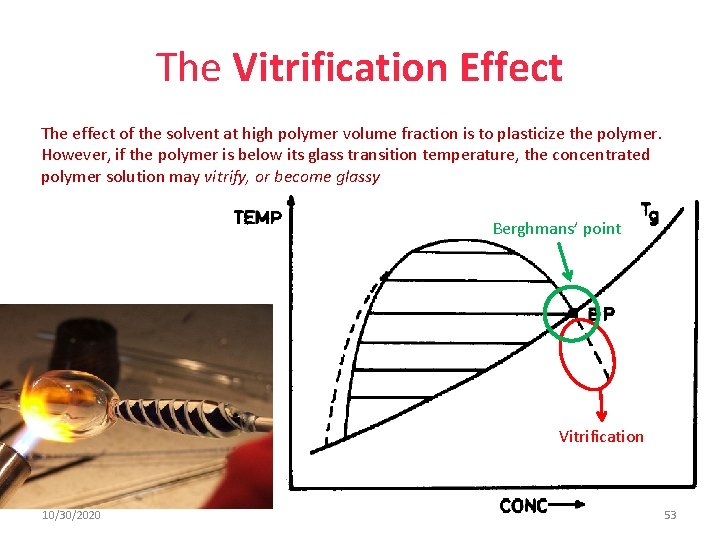
The Vitrification Effect The effect of the solvent at high polymer volume fraction is to plasticize the polymer. However, if the polymer is below its glass transition temperature, the concentrated polymer solution may vitrify, or become glassy Berghmans’ point Vitrification 10/30/2020 53

Polymer Blends 10/30/2020 54

POLYMER–POLYMER Miscibility O P Y P O R L L M Y E E M R PHASE SEPARATION 10/30/2020 55

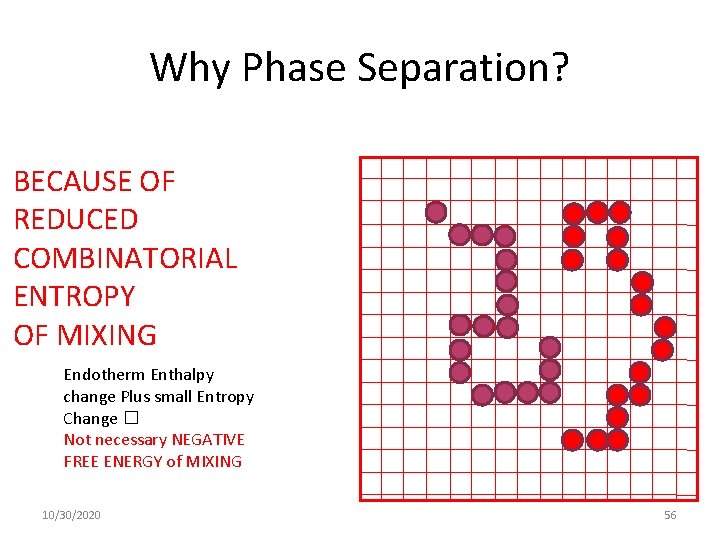
Why Phase Separation? BECAUSE OF REDUCED COMBINATORIAL ENTROPY OF MIXING Endotherm Enthalpy change Plus small Entropy Change � Not necessary NEGATIVE FREE ENERGY of MIXING 10/30/2020 56

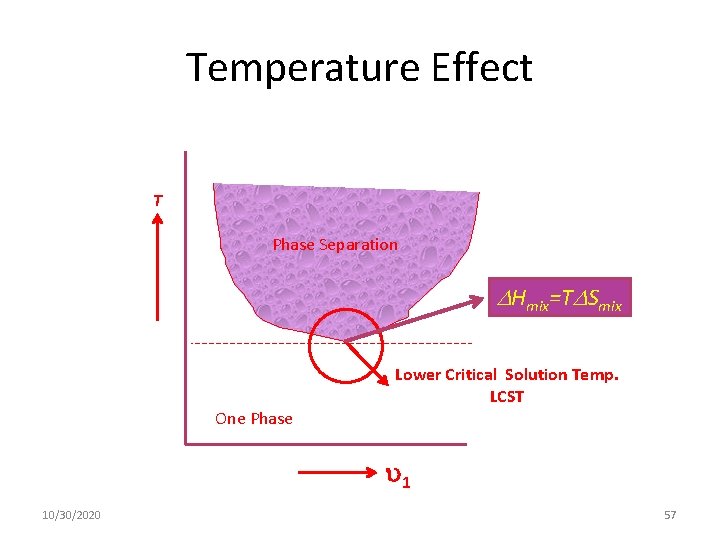
Temperature Effect T Phase Separation Hmix=T Smix Lower Critical Solution Temp. LCST One Phase 1 10/30/2020 57

PHASE DIAGRAMS Spinodal Lines Binodal Line 10/30/2020 58

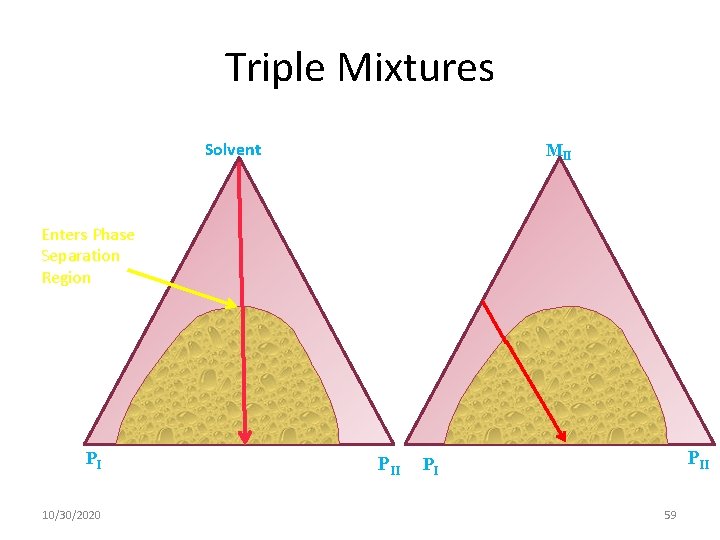
Triple Mixtures Solvent MII Enters Phase Separation Region PI 10/30/2020 PII PI 59

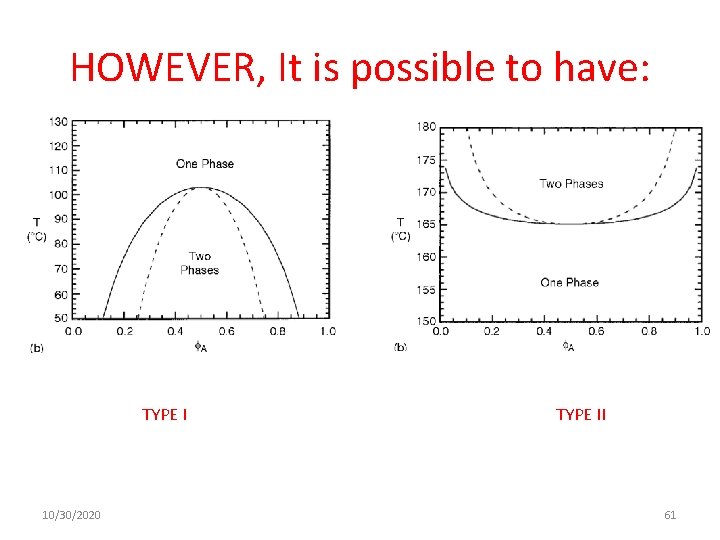
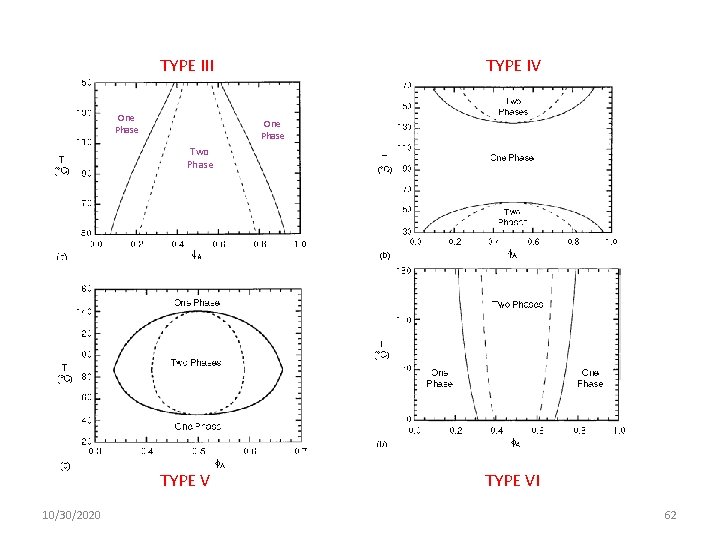
HOWEVER, It is possible to have: TYPE I 10/30/2020 TYPE II 61

TYPE III One TYPE IV One Phase Two Phase TYPE V 10/30/2020 TYPE VI 62

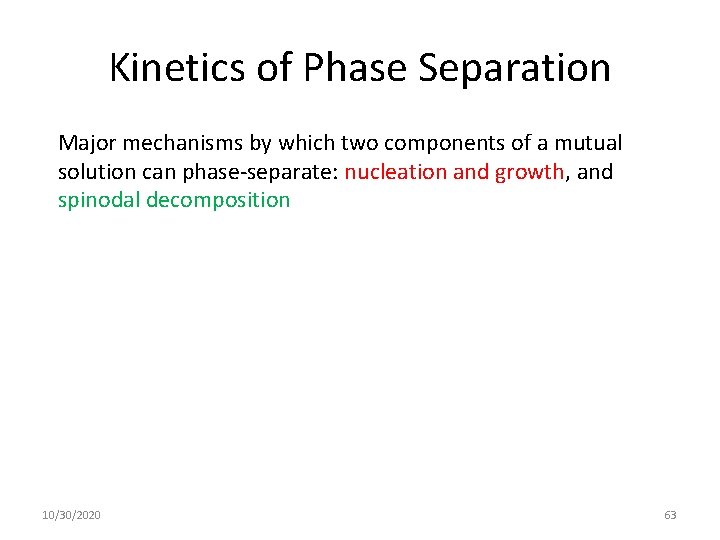
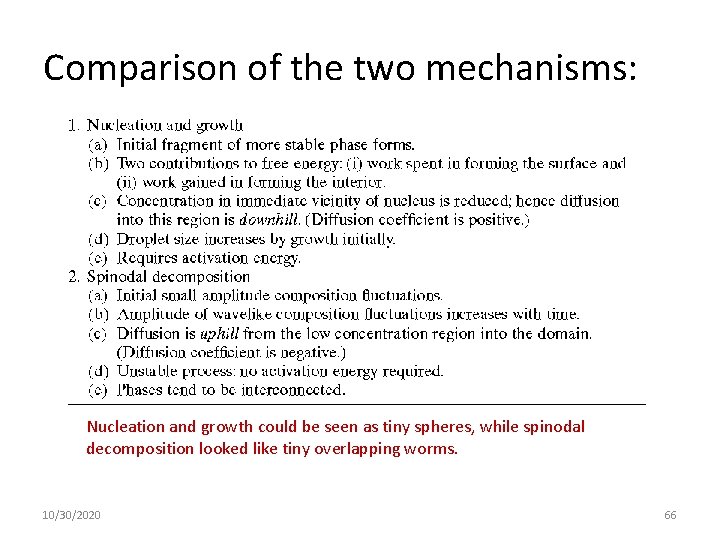
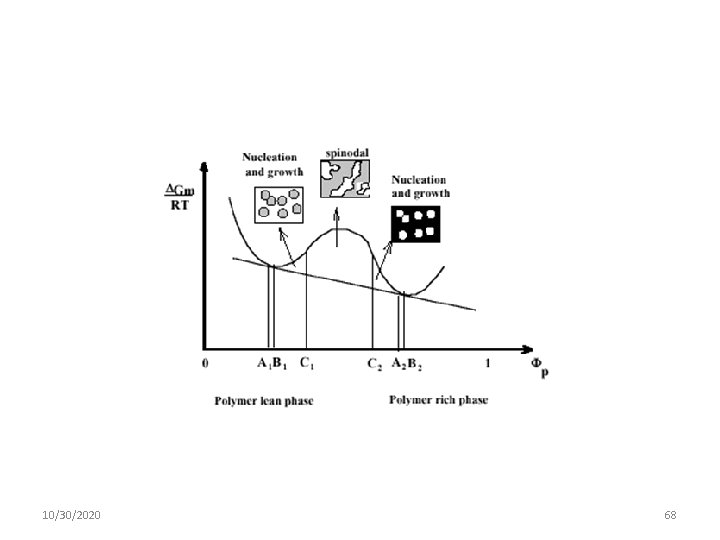
Kinetics of Phase Separation Major mechanisms by which two components of a mutual solution can phase-separate: nucleation and growth, and spinodal decomposition 10/30/2020 63

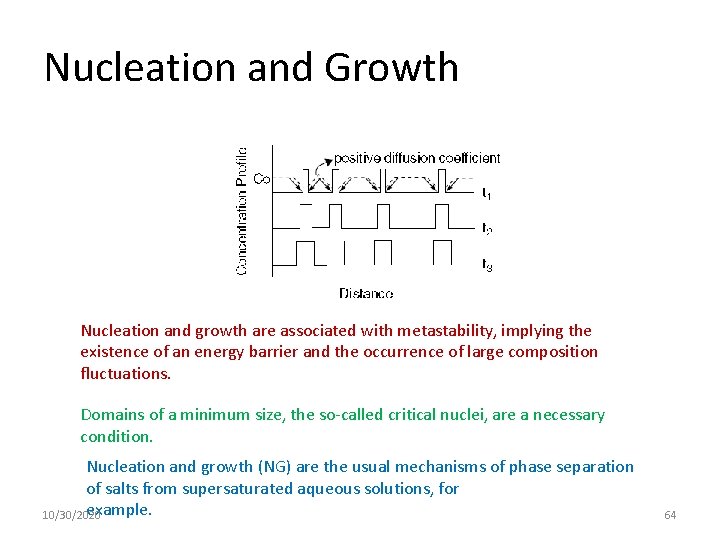
Nucleation and Growth Nucleation and growth are associated with metastability, implying the existence of an energy barrier and the occurrence of large composition fluctuations. Domains of a minimum size, the so-called critical nuclei, are a necessary condition. Nucleation and growth (NG) are the usual mechanisms of phase separation of salts from supersaturated aqueous solutions, for example. 10/30/2020 64

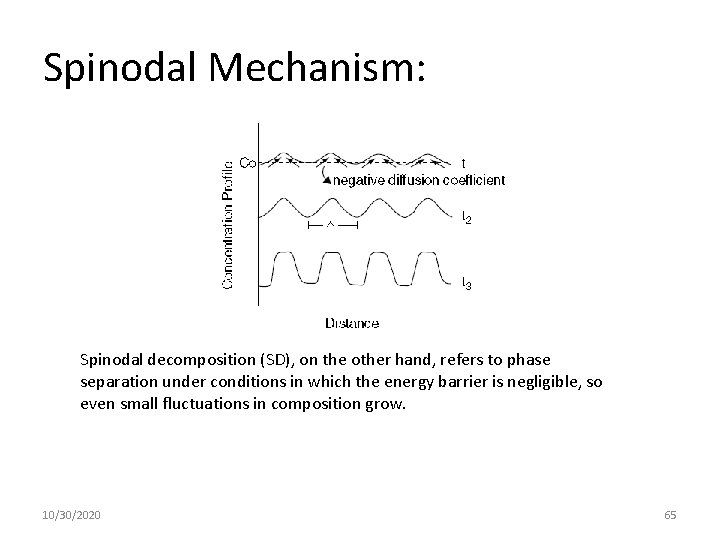
Spinodal Mechanism: Spinodal decomposition (SD), on the other hand, refers to phase separation under conditions in which the energy barrier is negligible, so even small fluctuations in composition grow. 10/30/2020 65

Comparison of the two mechanisms: Nucleation and growth could be seen as tiny spheres, while spinodal decomposition looked like tiny overlapping worms. 10/30/2020 66

Investigation under Microscope Which kind of phase separation does the picture show? 10/30/2020 67

10/30/2020 68

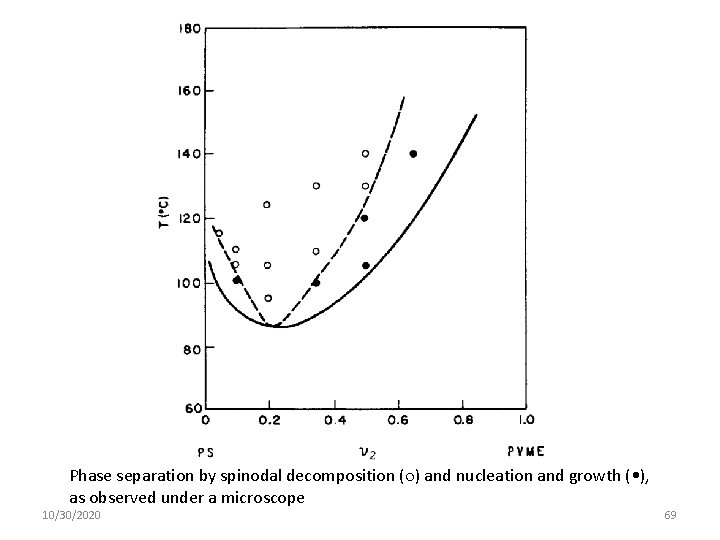
Phase separation by spinodal decomposition ( ) and nucleation and growth ( • ), as observed under a microscope 10/30/2020 69

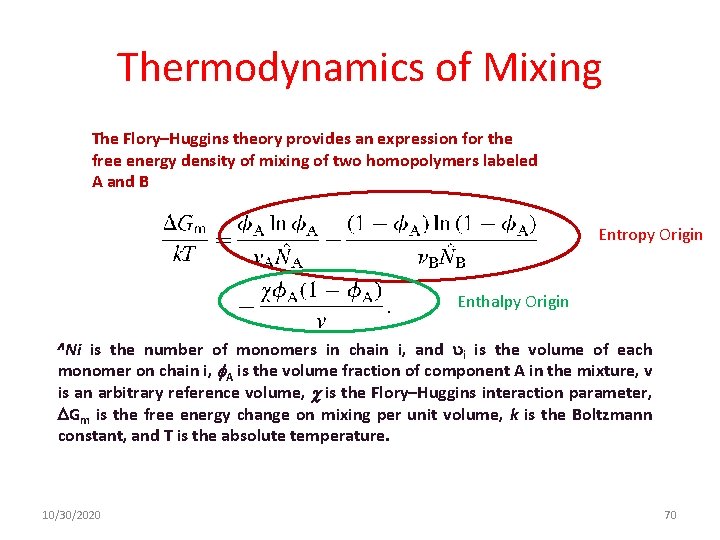
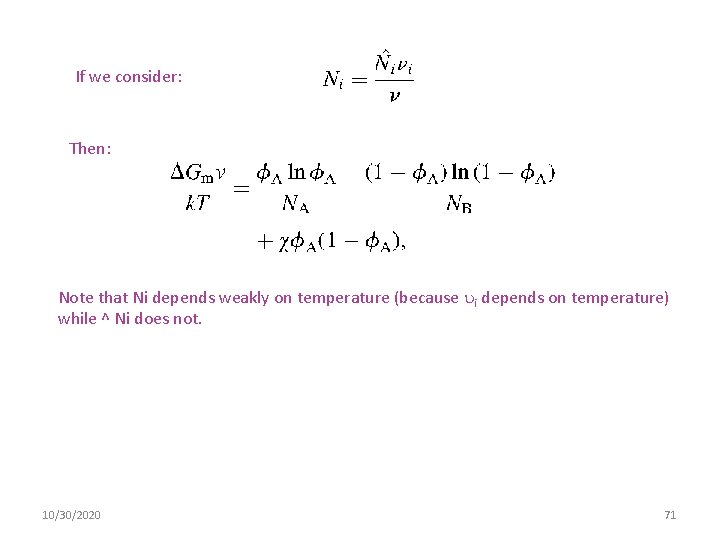
Thermodynamics of Mixing The Flory–Huggins theory provides an expression for the free energy density of mixing of two homopolymers labeled A and B Entropy Origin Enthalpy Origin is the number of monomers in chain i, and i is the volume of each monomer on chain i, A is the volume fraction of component A in the mixture, v is an arbitrary reference volume, is the Flory–Huggins interaction parameter, Gm is the free energy change on mixing per unit volume, k is the Boltzmann constant, and T is the absolute temperature. Ni 10/30/2020 70

If we consider: Then: Note that Ni depends weakly on temperature (because i depends on temperature) while ^ Ni does not. 10/30/2020 71

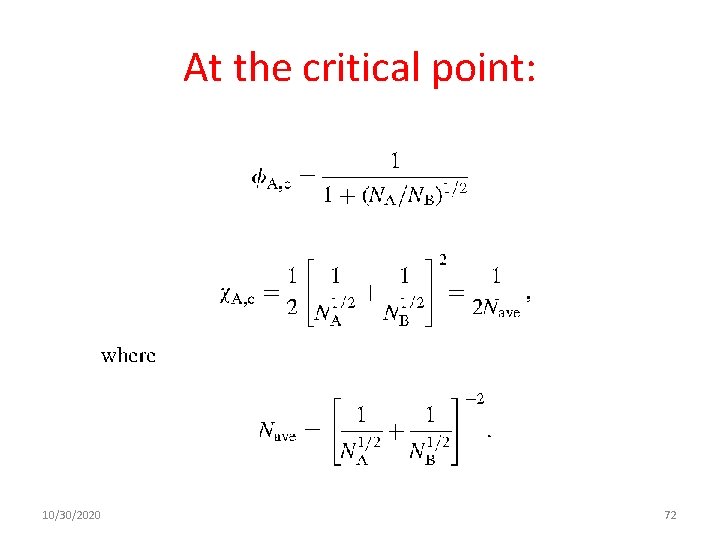
At the critical point: 10/30/2020 72

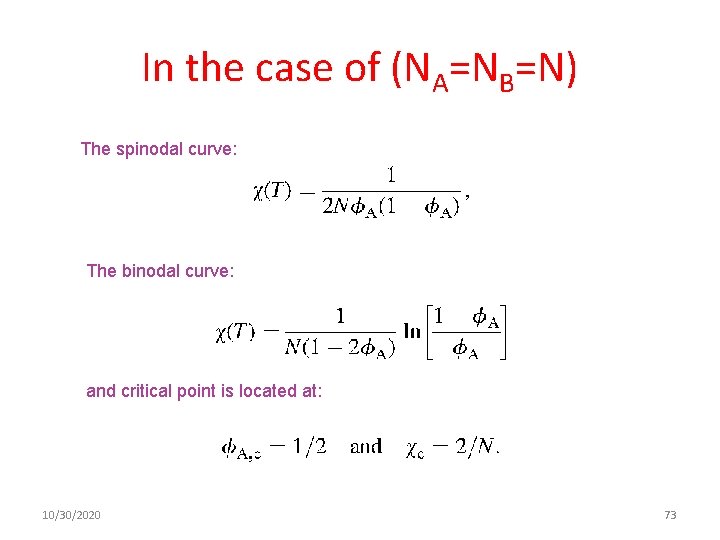
In the case of (NA=NB=N) The spinodal curve: The binodal curve: and critical point is located at: 10/30/2020 73

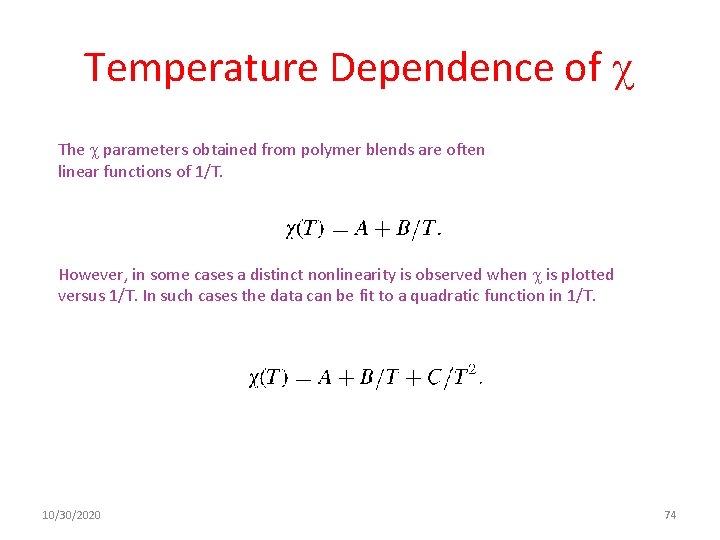
Temperature Dependence of The parameters obtained from polymer blends are often linear functions of 1/T. However, in some cases a distinct nonlinearity is observed when is plotted versus 1/T. In such cases the data can be fit to a quadratic function in 1/T. 10/30/2020 74

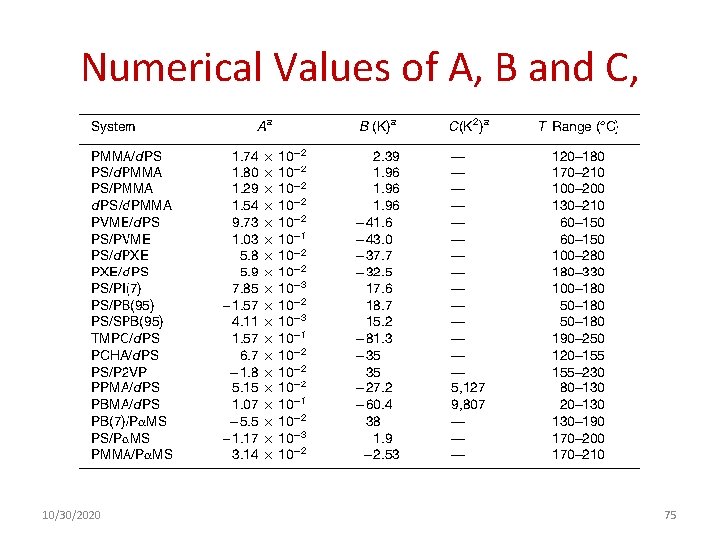
Numerical Values of A, B and C, 10/30/2020 75

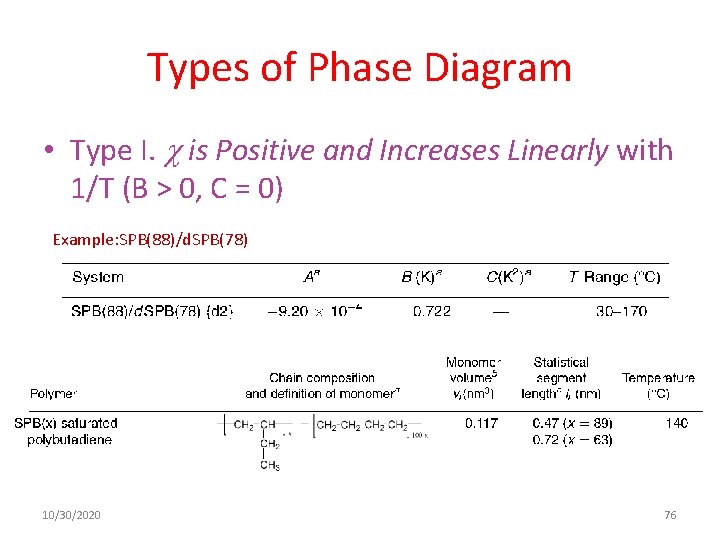
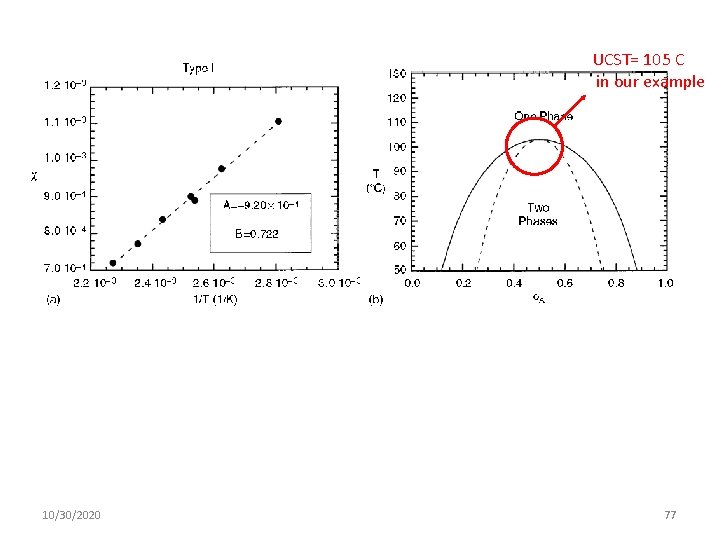
Types of Phase Diagram • Type I. is Positive and Increases Linearly with 1/T (B > 0, C = 0) Example: SPB(88)/d. SPB(78) 10/30/2020 76

UCST= 105 C in our example 10/30/2020 77

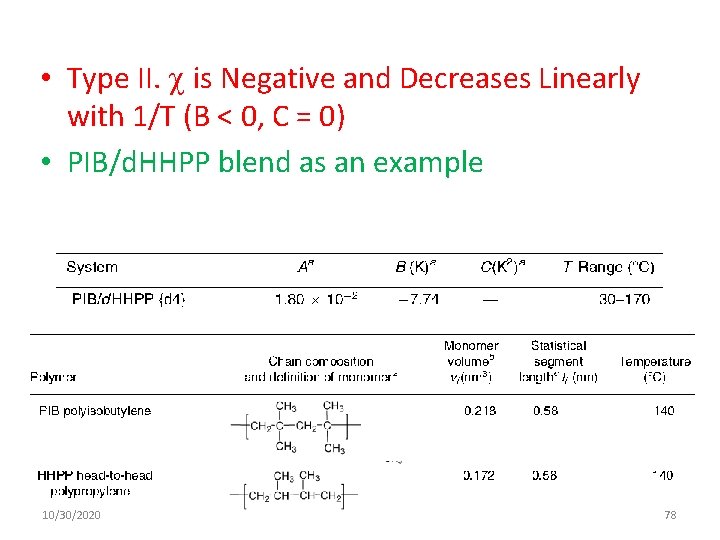
• Type II. is Negative and Decreases Linearly with 1/T (B < 0, C = 0) • PIB/d. HHPP blend as an example 10/30/2020 78

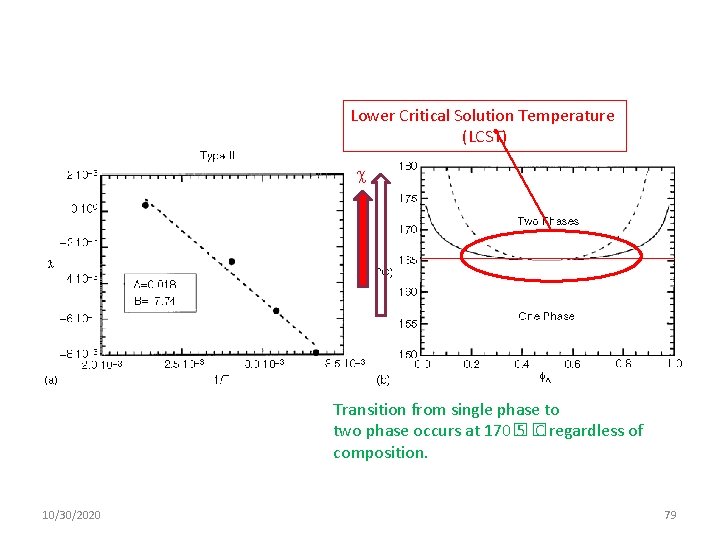
Lower Critical Solution Temperature (LCST) Transition from single phase to two phase occurs at 170� 5� C regardless of composition. 10/30/2020 79

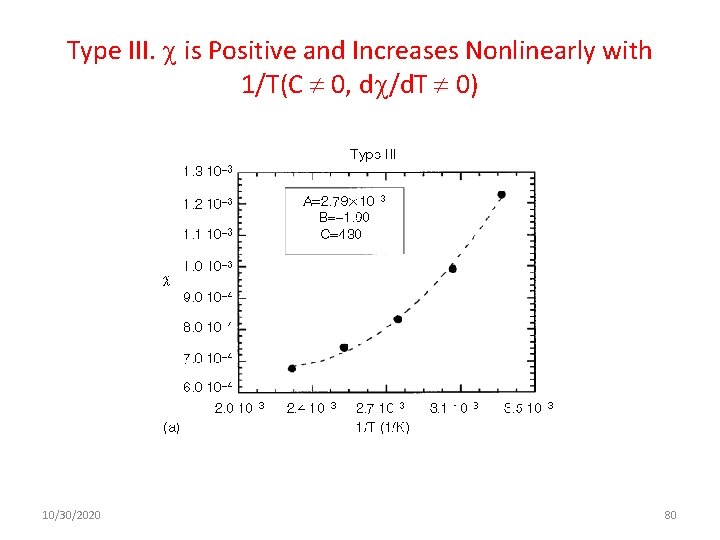
Type III. is Positive and Increases Nonlinearly with 1/T(C 0, d /d. T 0) 10/30/2020 80

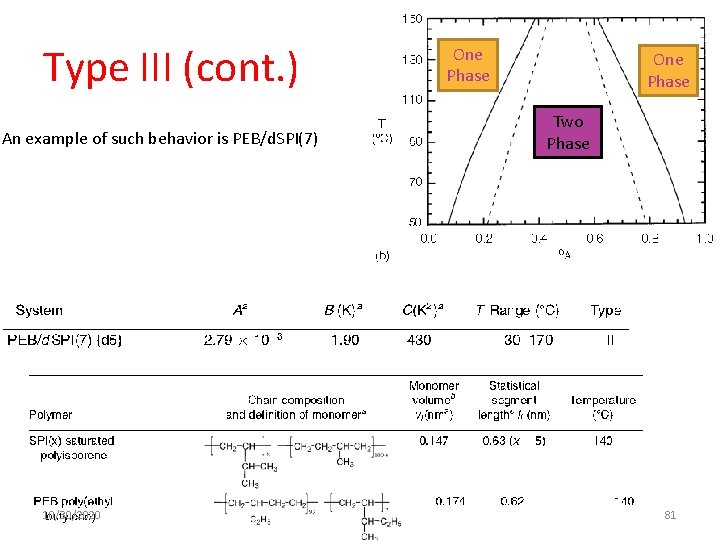
Type III (cont. ) An example of such behavior is PEB/d. SPI(7) 10/30/2020 One Phase Two Phase 81

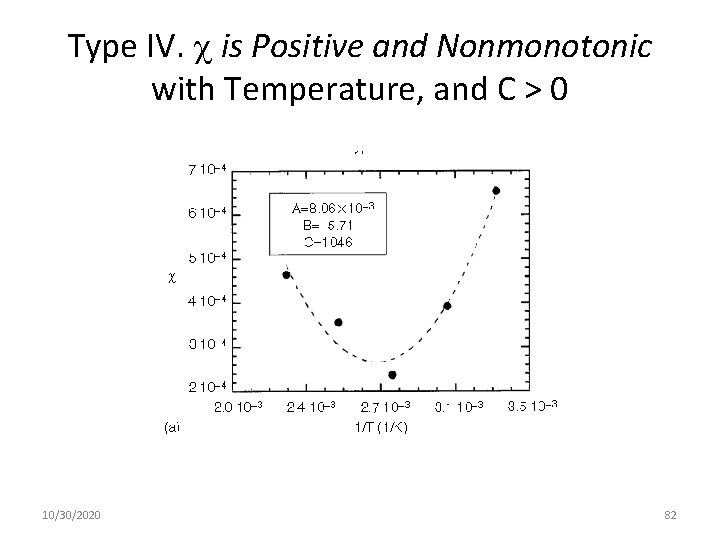
Type IV. is Positive and Nonmonotonic with Temperature, and C > 0 10/30/2020 82

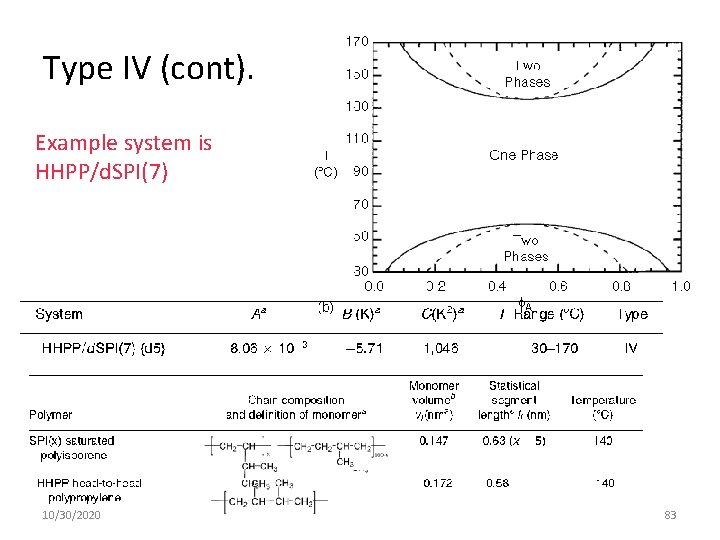
Type IV (cont). Example system is HHPP/d. SPI(7) 10/30/2020 83

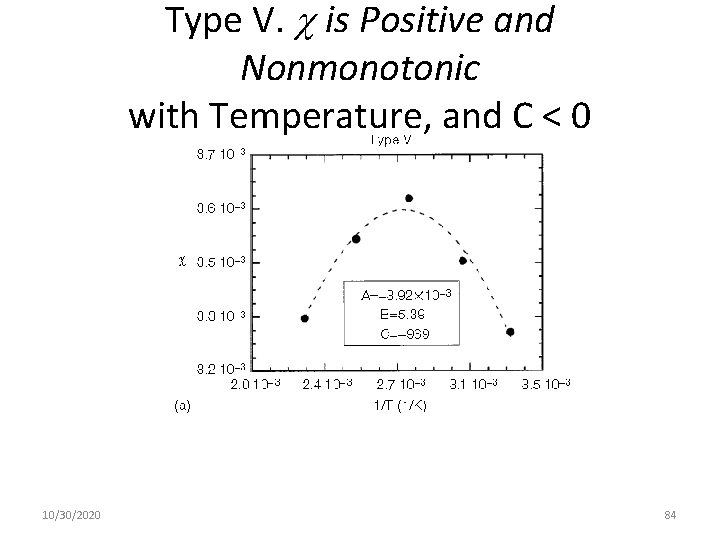
Type V. is Positive and Nonmonotonic with Temperature, and C < 0 10/30/2020 84

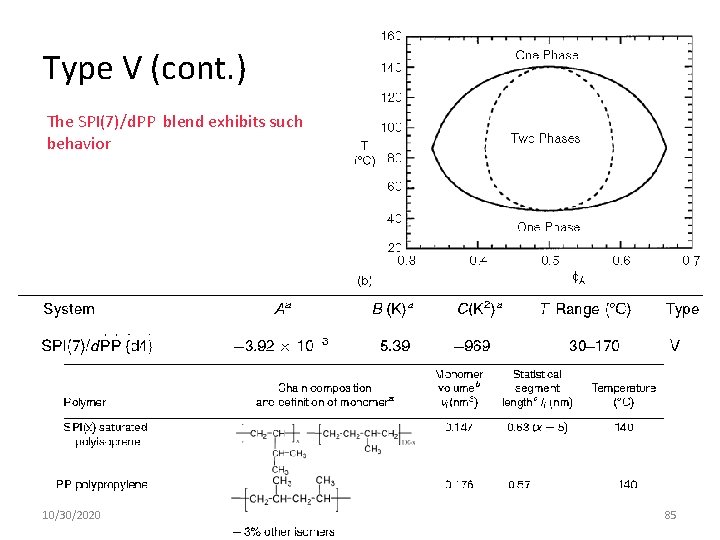
Type V (cont. ) The SPI(7)/d. PP blend exhibits such behavior 10/30/2020 85

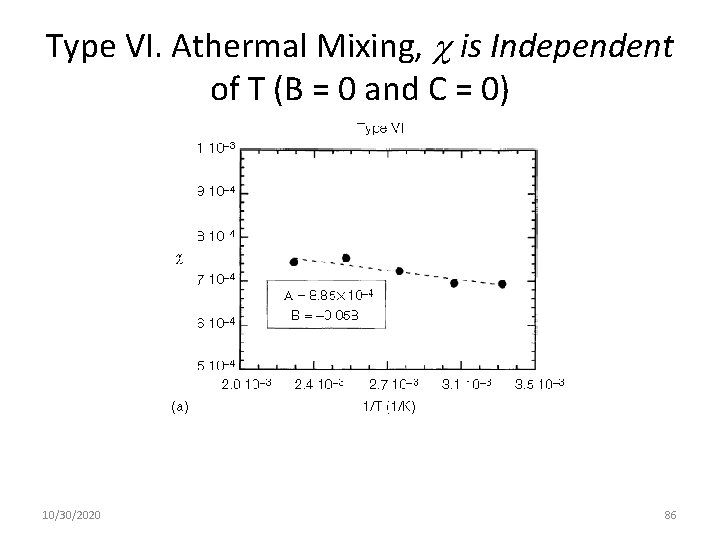
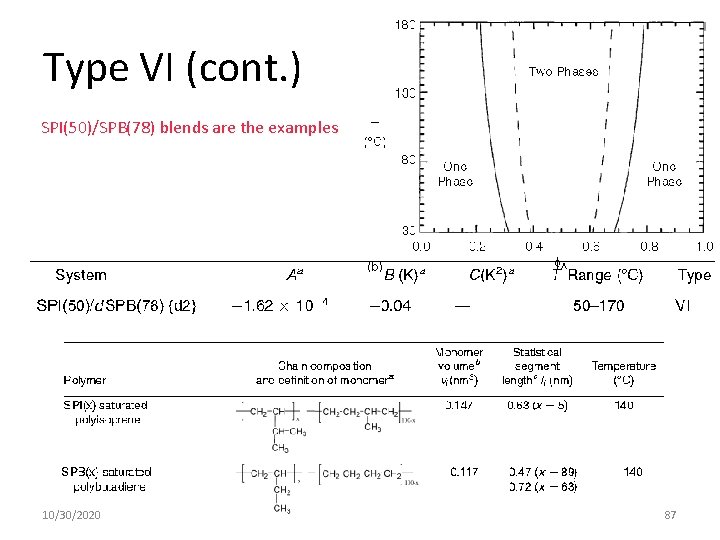
Type VI. Athermal Mixing, is Independent of T (B = 0 and C = 0) 10/30/2020 86

Type VI (cont. ) SPI(50)/SPB(78) blends are the examples 10/30/2020 87

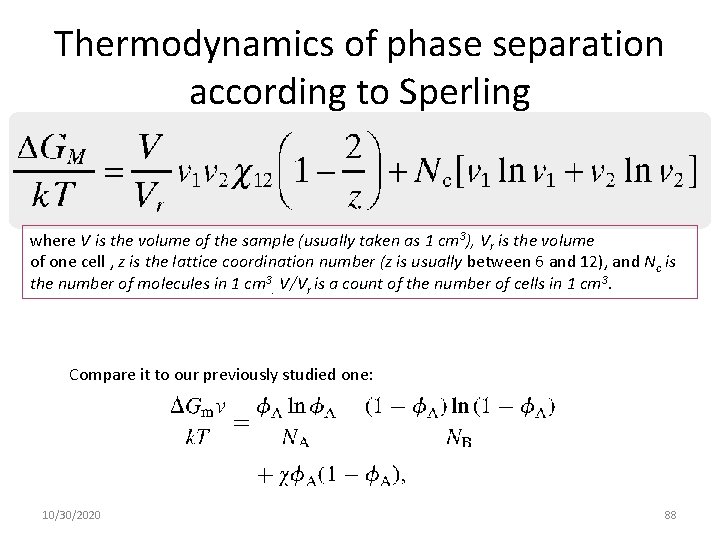
Thermodynamics of phase separation according to Sperling where V is the volume of the sample (usually taken as 1 cm 3), Vr is the volume of one cell , z is the lattice coordination number (z is usually between 6 and 12), and Nc is the number of molecules in 1 cm 3. V/Vr is a count of the number of cells in 1 cm 3. Compare it to our previously studied one: 10/30/2020 88

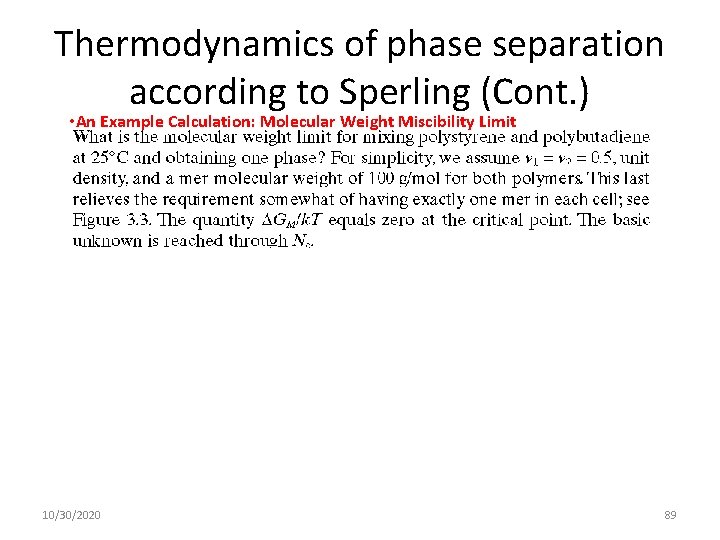
Thermodynamics of phase separation according to Sperling (Cont. ) • An Example Calculation: Molecular Weight Miscibility Limit 10/30/2020 89

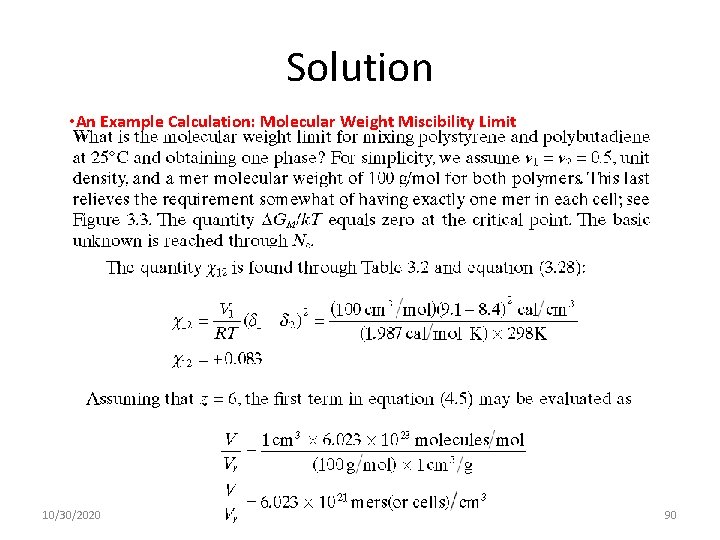
Solution • An Example Calculation: Molecular Weight Miscibility Limit 10/30/2020 90

10/30/2020 91

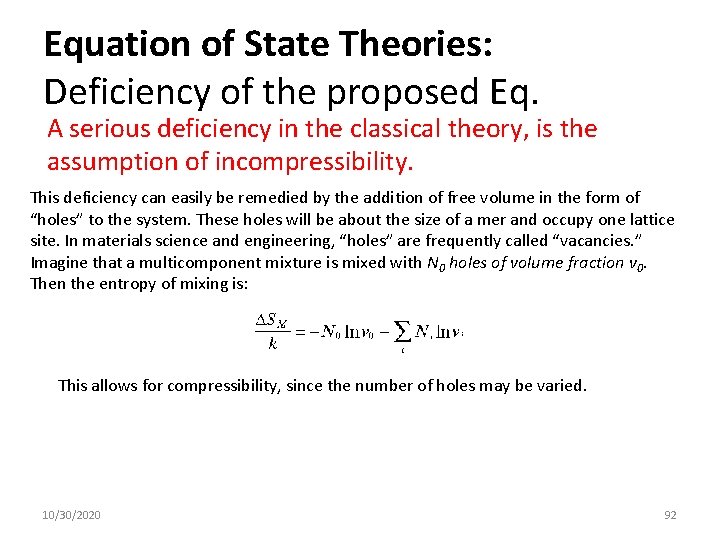
Equation of State Theories: Deficiency of the proposed Eq. A serious deficiency in the classical theory, is the assumption of incompressibility. This deficiency can easily be remedied by the addition of free volume in the form of “holes” to the system. These holes will be about the size of a mer and occupy one lattice site. In materials science and engineering, “holes” are frequently called “vacancies. ” Imagine that a multicomponent mixture is mixed with N 0 holes of volume fraction v 0. Then the entropy of mixing is: This allows for compressibility, since the number of holes may be varied. 10/30/2020 92

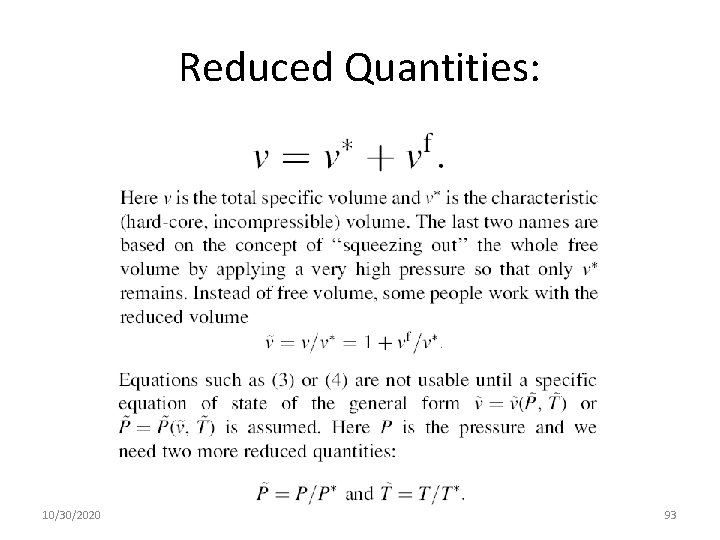
Reduced Quantities: 10/30/2020 93

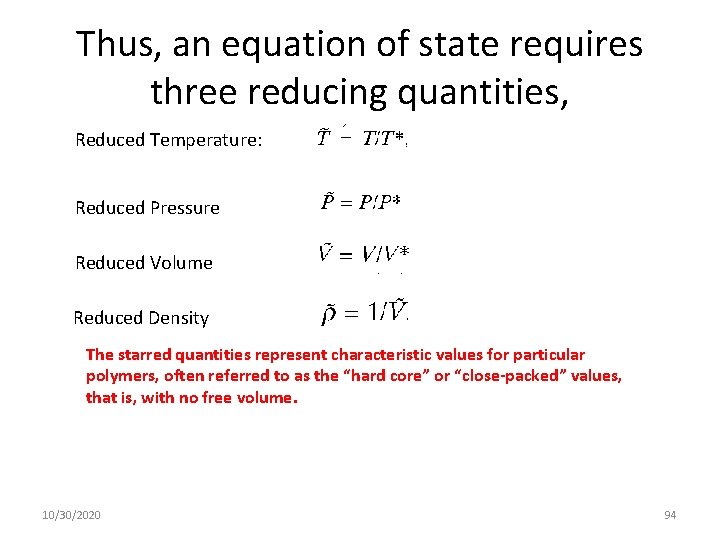
Thus, an equation of state requires three reducing quantities, Reduced Temperature: Reduced Pressure Reduced Volume Reduced Density The starred quantities represent characteristic values for particular polymers, often referred to as the “hard core” or “close-packed” values, that is, with no free volume. 10/30/2020 94

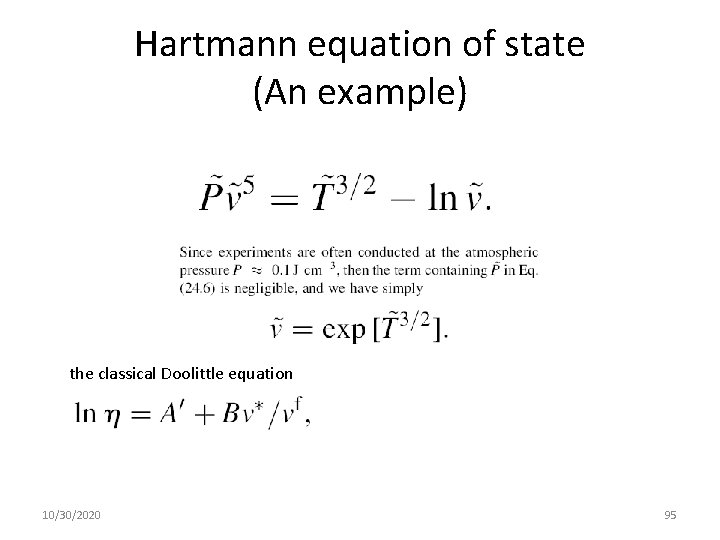
Hartmann equation of state (An example) the classical Doolittle equation 10/30/2020 95

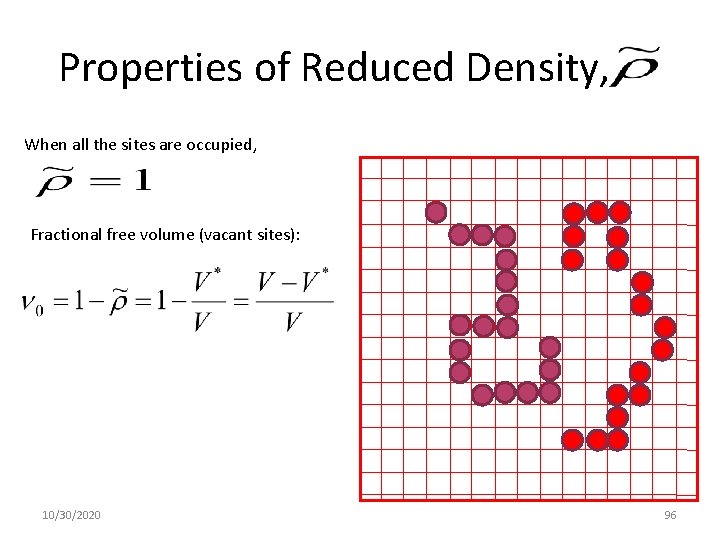
Properties of Reduced Density, When all the sites are occupied, Fractional free volume (vacant sites): 10/30/2020 96

The Entropy of Mixing, Now! The entropy of mixing vacant sites with the molecules in equation of state terminology is given by When all the sites are occupied, = 1, and the right hand side is zero. where the quantity * is a van der Waals type of energy of interaction. Note that ˜ = ˜ (P, T); ˜ 1 as T 0; ˜ 1 as P �. 10/30/2020 97

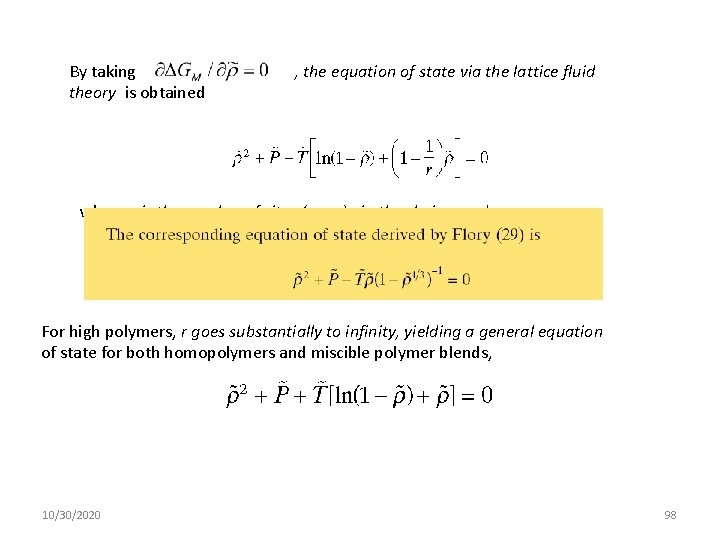
By taking theory is obtained , the equation of state via the lattice fluid where r is the number of sites (mers), in the chains, and For high polymers, r goes substantially to infinity, yielding a general equation of state for both homopolymers and miscible polymer blends, 10/30/2020 98

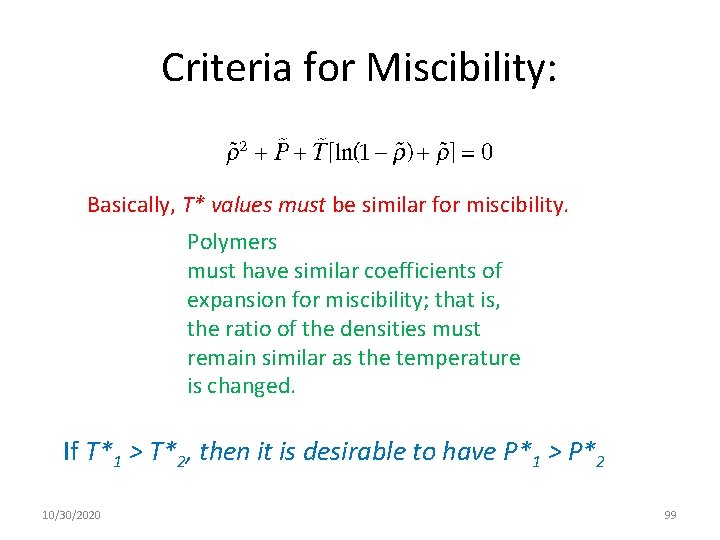
Criteria for Miscibility: Basically, T* values must be similar for miscibility. Polymers must have similar coefficients of expansion for miscibility; that is, the ratio of the densities must remain similar as the temperature is changed. If T*1 > T*2, then it is desirable to have P*1 > P*2 10/30/2020 99

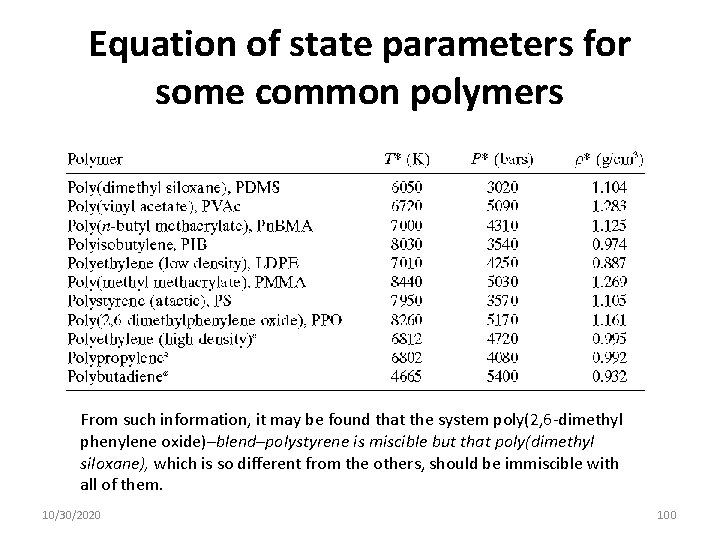
Equation of state parameters for some common polymers From such information, it may be found that the system poly(2, 6 -dimethyl phenylene oxide)–blend–polystyrene is miscible but that poly(dimethyl siloxane), which is so different from the others, should be immiscible with all of them. 10/30/2020 100

Very Important Conclusions: • For the Flory–Huggins theory of incompressible polymer mixtures, the small entropy of mixing is dominated by the heat of mixing, leading to the conclusion that the heat of mixing must be zero or negative to induce miscibility. • When two compressible polymers are mixed together, negative heats of mixing cause a negative volume change. • Since reducing the volume of the system reduces the number of holes, the entropy of mixing change is negative. • At high enough temperatures, the unfavorable entropy change associated with the densification of the mixture becomes prohibitive, that is, T S > H, and the mixture phase-separates. 10/30/2020 101

Very Important Conclusions: • The two-phase system has a larger volume as the holes are reintroduced, and hence a larger positive contribution to the entropy. • Significantly differing coefficients of expansion contribute to phase separation. • In virtually all polymer–polymer systems exhibiting critical phenomena, both H and T S are relatively small quantities. Thus relatively modest changes in either the enthalpy or the entropy alter the phase diagram significantly. 10/30/2020 102

Let’s try softwares Applet by Maye’s group 10/30/2020 103

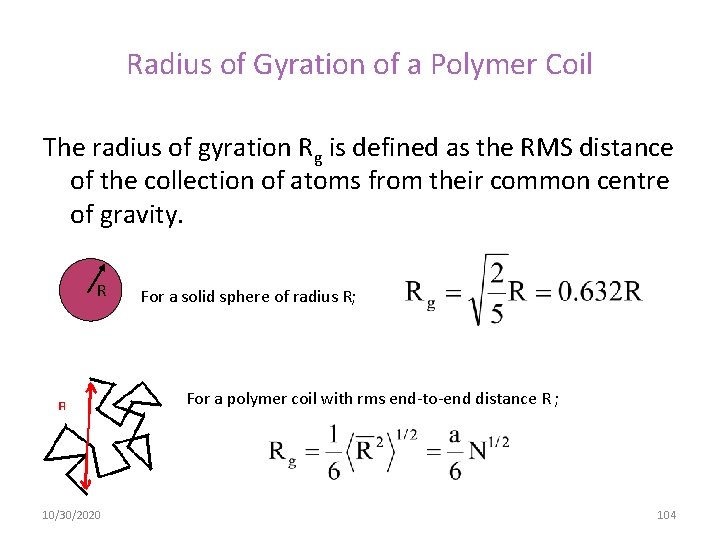
Radius of Gyration of a Polymer Coil The radius of gyration Rg is defined as the RMS distance of the collection of atoms from their common centre of gravity. R For a solid sphere of radius R; For a polymer coil with rms end-to-end distance R ; 10/30/2020 104

The excluded volume effect • Steric hindrance on short distances limits the number of conformations • At longer distances we have a topological constraint – the self avoiding walk – or the excluded volume effect: Instead of <R 2>1/2=a. N 1/2 we will have <R 2>1/2=a. Nν where v>0. 5 Experiments tells us that in general: v~0. 6 Why? 10/30/2020 105

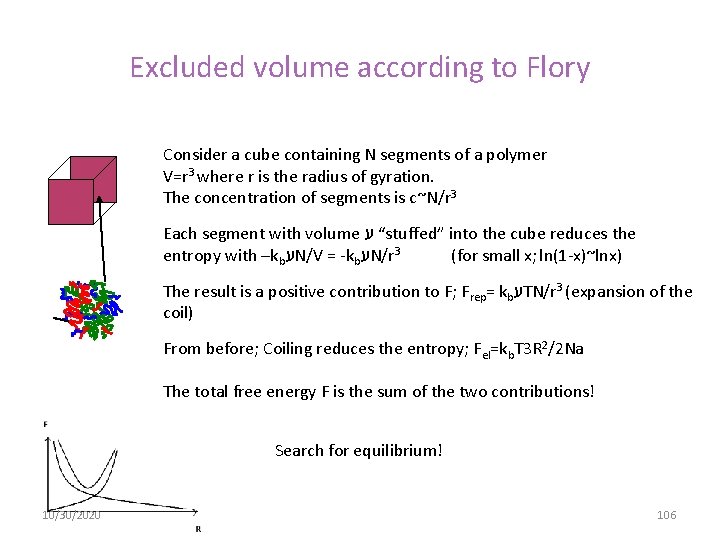
Excluded volume according to Flory Consider a cube containing N segments of a polymer V=r 3 where r is the radius of gyration. The concentration of segments is c~N/r 3 Each segment with volume “ ע stuffed” into the cube reduces the entropy with –kb ע N/V = -kb ע N/r 3 (for small x; ln(1 -x)~lnx) The result is a positive contribution to F; Frep= kb ע TN/r 3 (expansion of the coil) From before; Coiling reduces the entropy; Fel=kb. T 3 R 2/2 Na The total free energy F is the sum of the two contributions! Search for equilibrium! 10/30/2020 106

Scaling Law Theories 10/30/2020 107

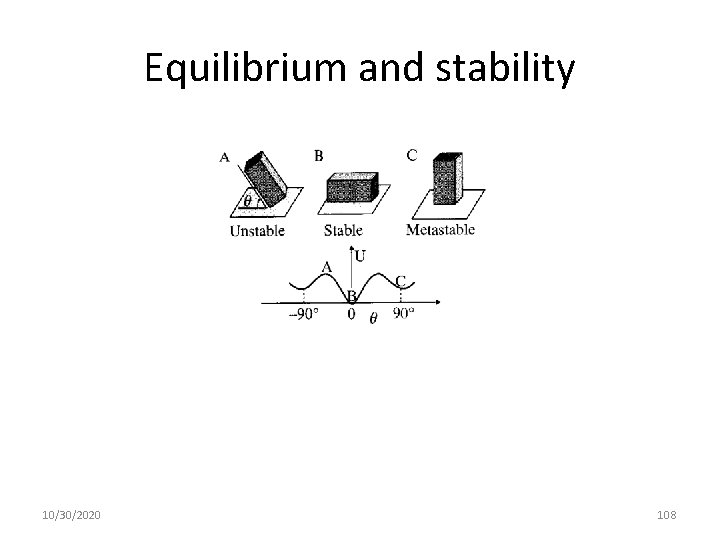
Equilibrium and stability 10/30/2020 108

Lever Rule Metastable Stable fraction fa of the volume of the material having composition Фα (and fraction fβ = 1 —fα having composition Фβ, 10/30/2020 109

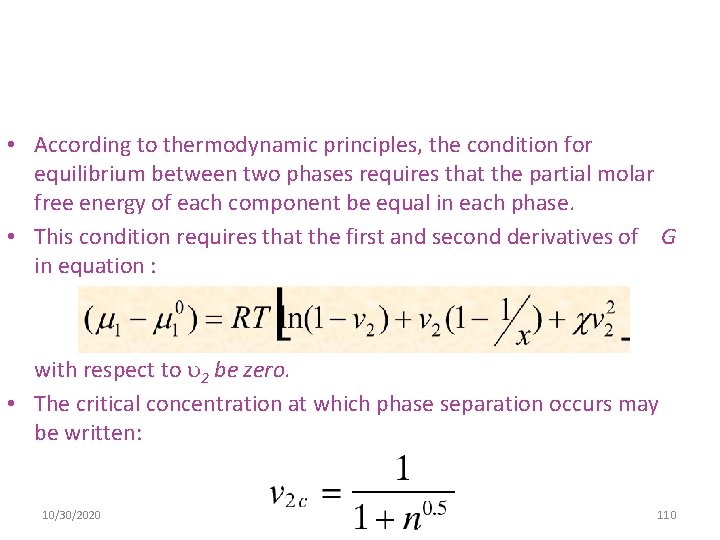
• According to thermodynamic principles, the condition for equilibrium between two phases requires that the partial molar free energy of each component be equal in each phase. • This condition requires that the first and second derivatives of G in equation : with respect to 2 be zero. • The critical concentration at which phase separation occurs may be written: 10/30/2020 110

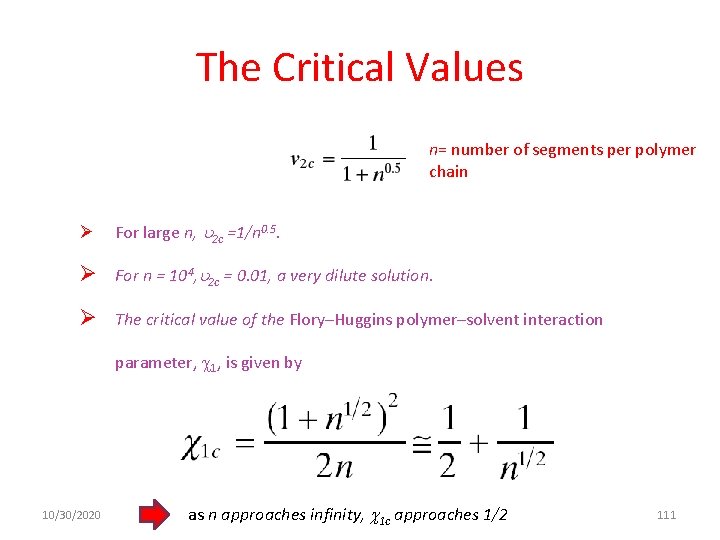
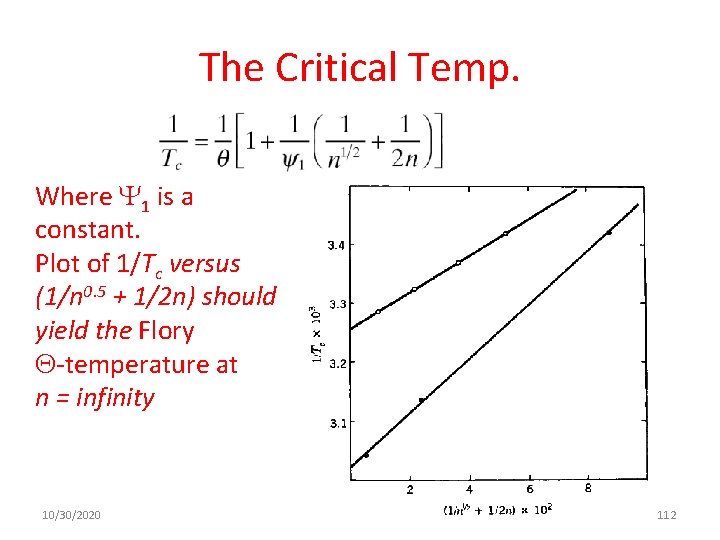
The Critical Values n= number of segments per polymer chain Ø For large n, 2 c =1/n 0. 5. Ø For n = 104, 2 c = 0. 01, a very dilute solution. Ø The critical value of the Flory–Huggins polymer–solvent interaction parameter, 1, is given by 10/30/2020 as n approaches infinity, 1 c approaches 1/2 111

The Critical Temp. Where 1 is a constant. Plot of 1/Tc versus (1/n 0. 5 + 1/2 n) should yield the Flory -temperature at n = infinity 10/30/2020 112

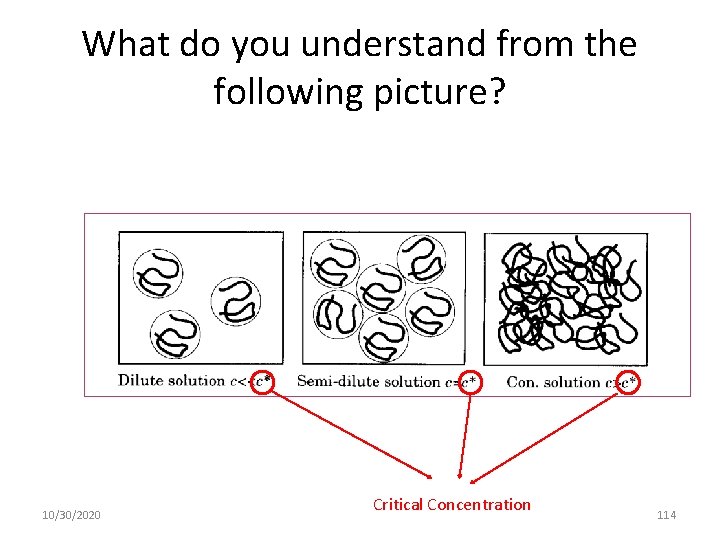
What do you understand from the following picture? 10/30/2020 Critical Concentration 114

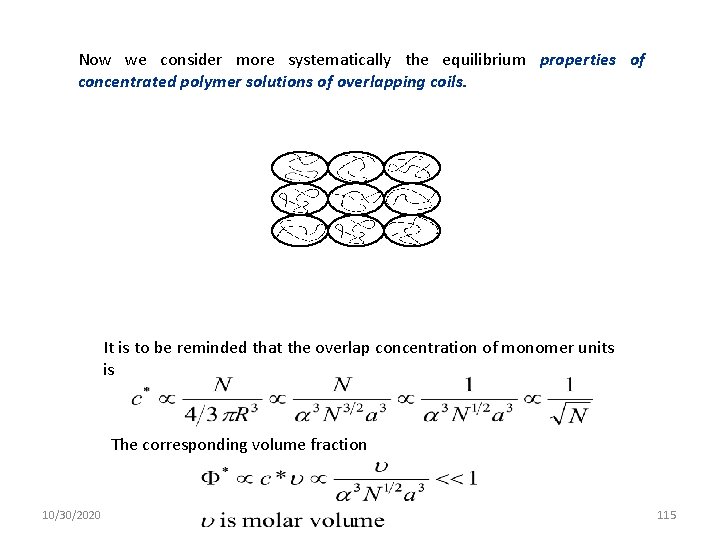
Now we consider more systematically the equilibrium properties of concentrated polymer solutions of overlapping coils. It is to be reminded that the overlap concentration of monomer units is The corresponding volume fraction 10/30/2020 115

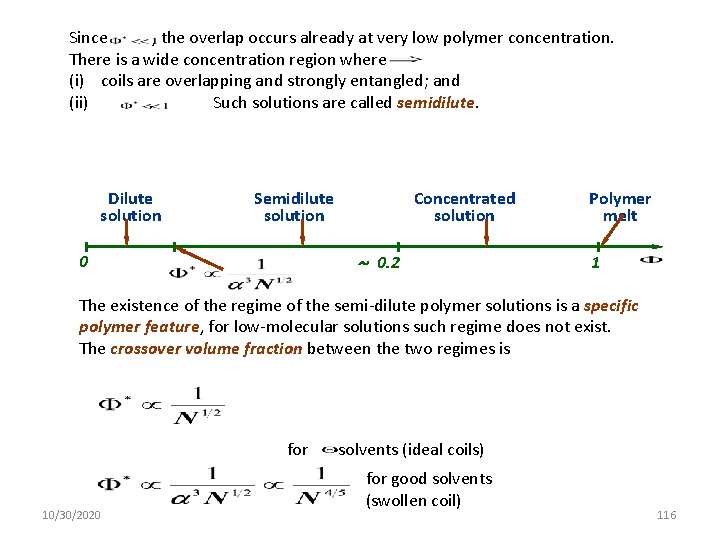
Since , the overlap occurs already at very low polymer concentration. There is a wide concentration region where (i) coils are overlapping and strongly entangled; and (ii). Such solutions are called semidilute. Dilute solution Semidilute solution Concentrated solution ~ 0. 2 0 Polymer melt 1 The existence of the regime of the semi-dilute polymer solutions is a specific polymer feature, for low-molecular solutions such regime does not exist. The crossover volume fraction between the two regimes is for 10/30/2020 -solvents (ideal coils) for good solvents (swollen coil) 116

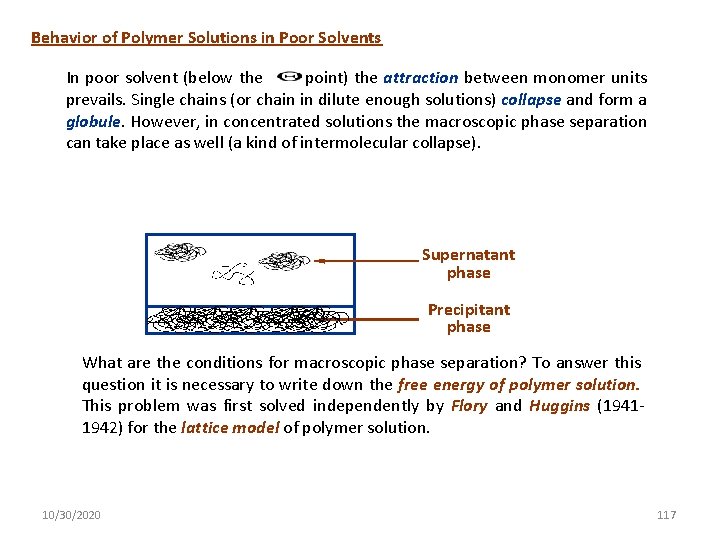
Behavior of Polymer Solutions in Poor Solvents In poor solvent (below the - point) the attraction between monomer units prevails. Single chains (or chain in dilute enough solutions) collapse and form a globule. However, in concentrated solutions the macroscopic phase separation can take place as well (a kind of intermolecular collapse). Supernatant phase Precipitant phase What are the conditions for macroscopic phase separation? To answer this question it is necessary to write down the free energy of polymer solution. This problem was first solved independently by Flory and Huggins (19411942) for the lattice model of polymer solution. 10/30/2020 117

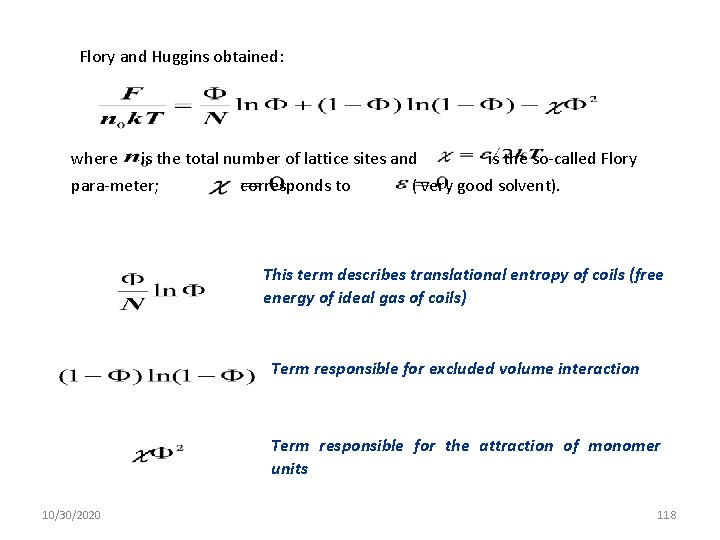
Flory and Huggins obtained: where is the total number of lattice sites and is the so-called Flory para-meter; corresponds to ( very good solvent). This term describes translational entropy of coils (free energy of ideal gas of coils) Term responsible for excluded volume interaction Term responsible for the attraction of monomer units 10/30/2020 118

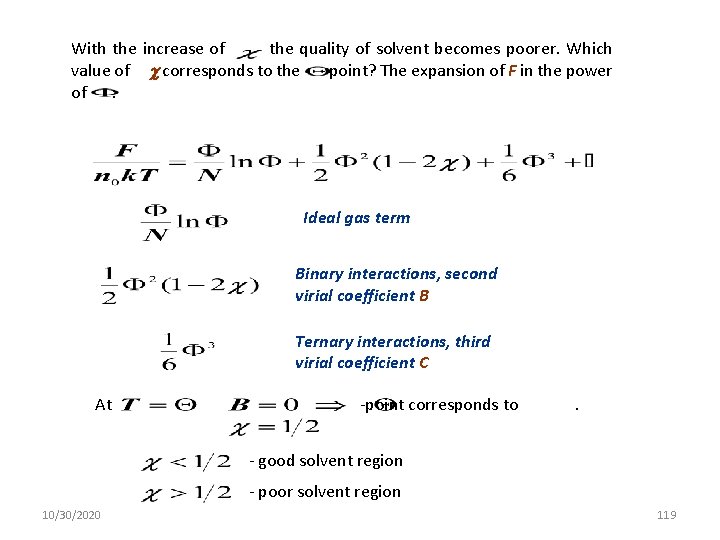
With the increase of the quality of solvent becomes poorer. Which value of corresponds to the - point? The expansion of F in the power of : Ideal gas term Binary interactions, second virial coefficient B Ternary interactions, third virial coefficient C At -point corresponds to . - good solvent region - poor solvent region 10/30/2020 119

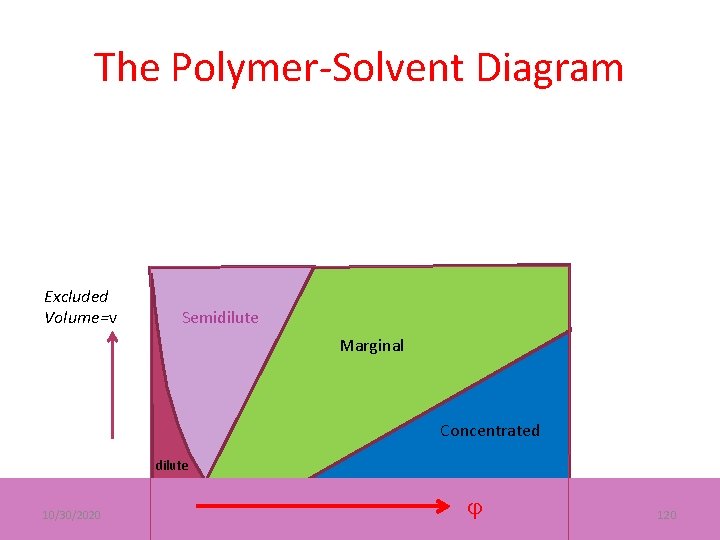
The Polymer-Solvent Diagram Excluded Volume=v Semidilute Marginal Concentrated dilute 10/30/2020 120

The Polymer-Solvent Diagram Semidilute Excluded Volume=v dilute Ideal 10/30/2020 Marginal Concentrated 121

Excluded Volume • A chain cannot cross itself in space. • Is a long-range interaction; eliminates conformations in which two widely separated segments would occupy the same space. 10/30/2020 123

Excluded Volume • At longer distances we have a topological constraint – the self avoiding walk – or the excluded volume effect: Instead of <r 2>1/2=a. N 1/2 we will have <r 2>1/2=a. Nν where v>0. 5 Experiments tells us that in general: v~0. 6 10/30/2020 124