Computer aided design CAD with Aspen Plus Javad











- Slides: 78

Computer aided design (CAD) with Aspen Plus Javad Ahmadpour j. ahmadpour@nit. ac. ir 1


Reactor Models Using Aspen Plus® V 9. 0

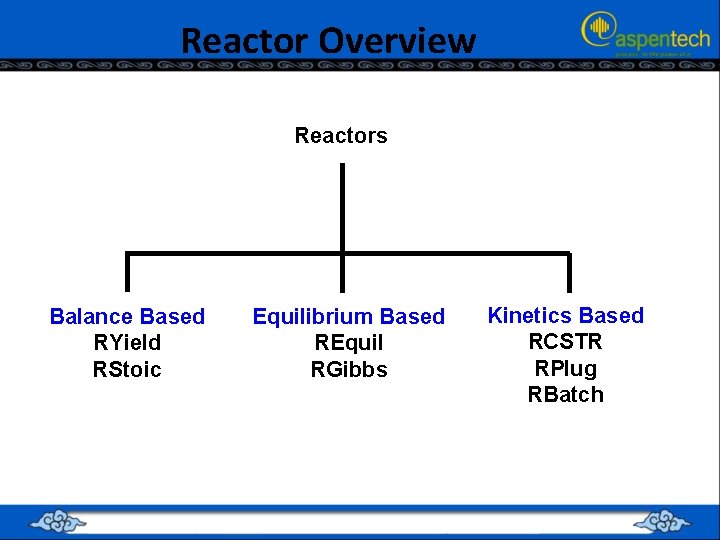
Reactor Overview Reactors Balance Based RYield RStoic Equilibrium Based REquil RGibbs Kinetics Based RCSTR RPlug RBatch

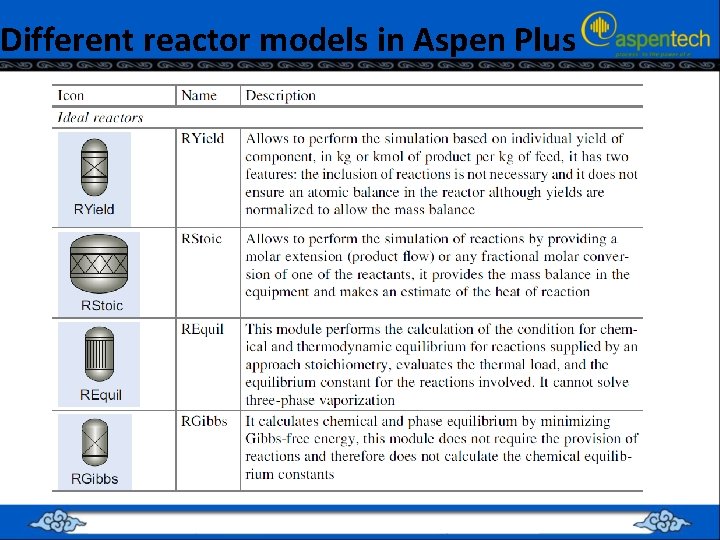
Different reactor models in Aspen Plus

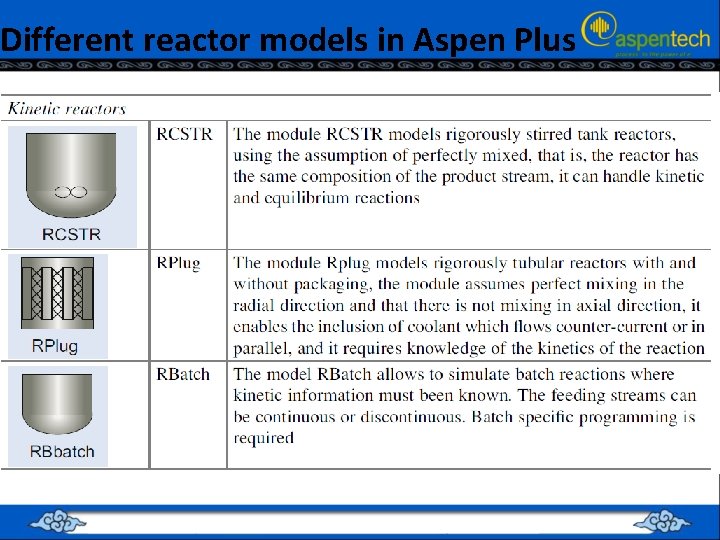
Different reactor models in Aspen Plus

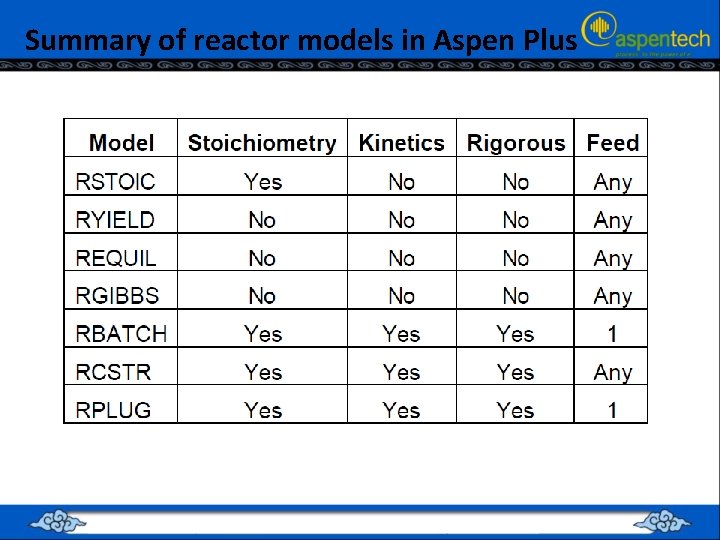
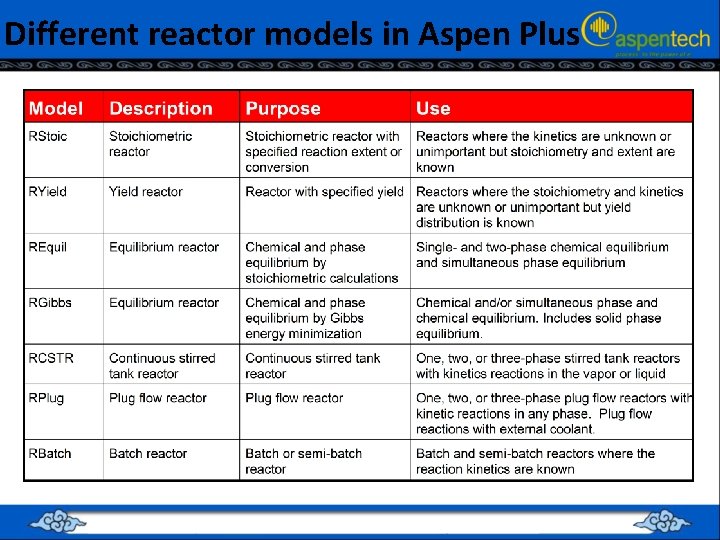
Summary of reactor models in Aspen Plus

Different reactor models in Aspen Plus

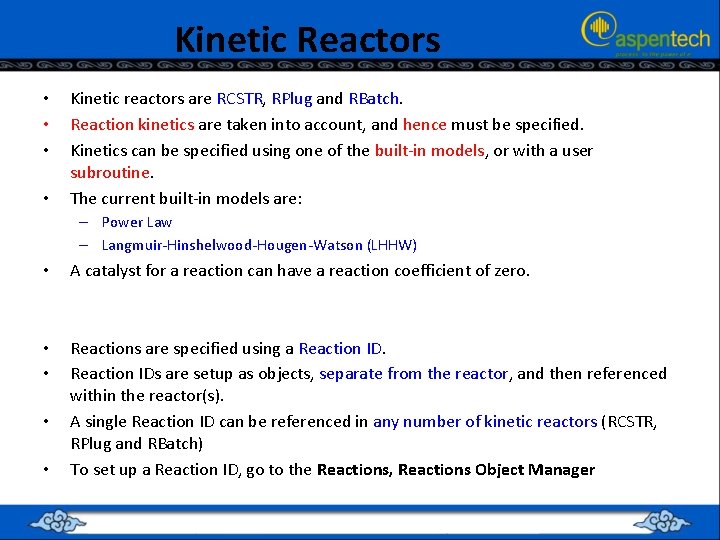
Kinetic Reactors • • Kinetic reactors are RCSTR, RPlug and RBatch. Reaction kinetics are taken into account, and hence must be specified. Kinetics can be specified using one of the built-in models, or with a user subroutine. The current built-in models are: – Power Law – Langmuir-Hinshelwood-Hougen-Watson (LHHW) • A catalyst for a reaction can have a reaction coefficient of zero. • • Reactions are specified using a Reaction IDs are setup as objects, separate from the reactor, and then referenced within the reactor(s). A single Reaction ID can be referenced in any number of kinetic reactors (RCSTR, RPlug and RBatch) To set up a Reaction ID, go to the Reactions, Reactions Object Manager • •

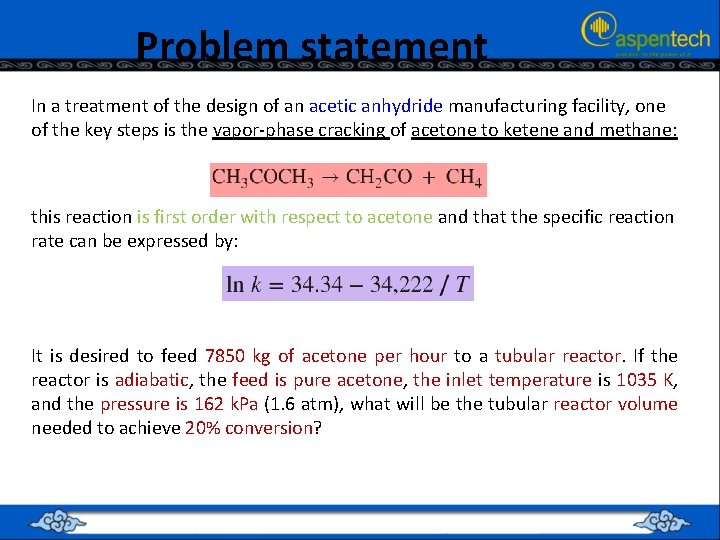
Problem statement In a treatment of the design of an acetic anhydride manufacturing facility, one of the key steps is the vapor-phase cracking of acetone to ketene and methane: this reaction is first order with respect to acetone and that the specific reaction rate can be expressed by: It is desired to feed 7850 kg of acetone per hour to a tubular reactor. If the reactor is adiabatic, the feed is pure acetone, the inlet temperature is 1035 K, and the pressure is 162 k. Pa (1. 6 atm), what will be the tubular reactor volume needed to achieve 20% conversion?

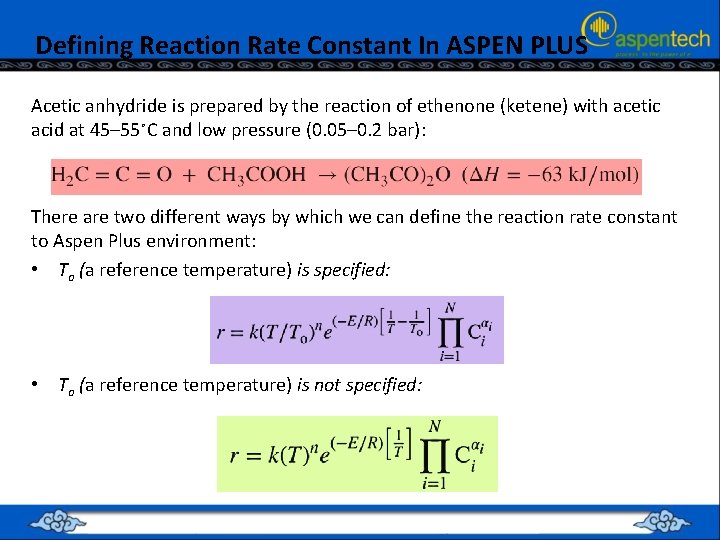
Defining Reaction Rate Constant In ASPEN PLUS Acetic anhydride is prepared by the reaction of ethenone (ketene) with acetic acid at 45– 55∘C and low pressure (0. 05– 0. 2 bar): There are two different ways by which we can define the reaction rate constant to Aspen Plus environment: • To (a reference temperature) is specified: • To (a reference temperature) is not specified:

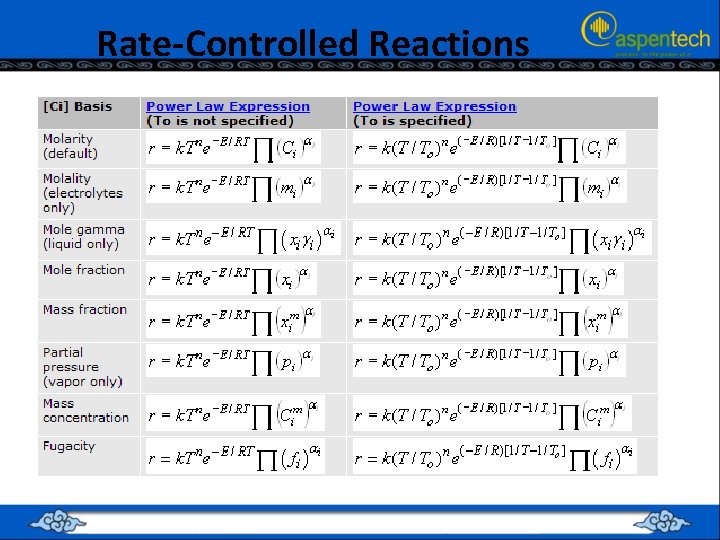
Rate-Controlled Reactions

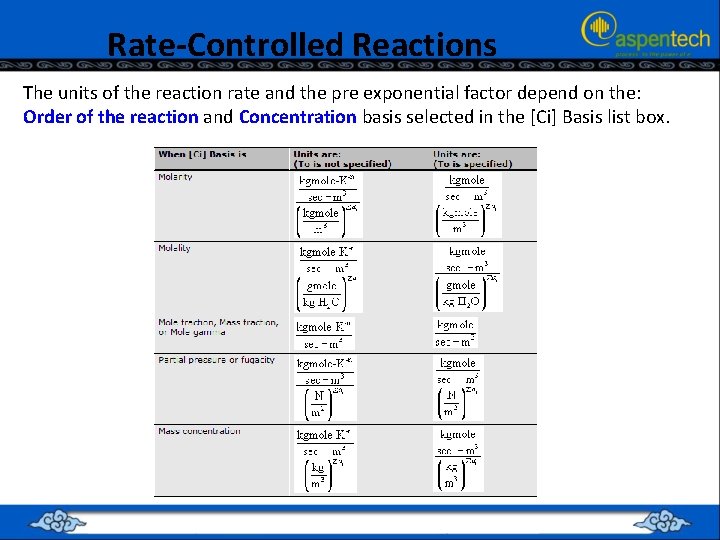
Rate-Controlled Reactions The units of the reaction rate and the pre exponential factor depend on the: Order of the reaction and Concentration basis selected in the [Ci] Basis list box.

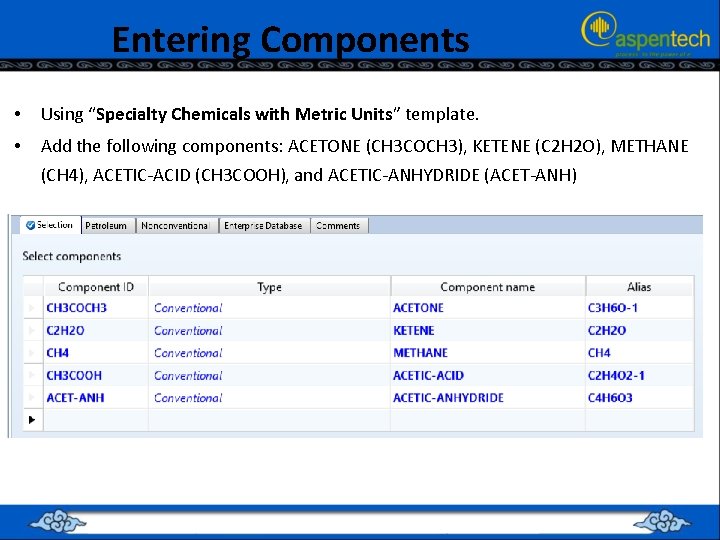
Entering Components • Using “Specialty Chemicals with Metric Units” template. • Add the following components: ACETONE (CH 3 COCH 3), KETENE (C 2 H 2 O), METHANE (CH 4), ACETIC-ACID (CH 3 COOH), and ACETIC-ANHYDRIDE (ACET-ANH)

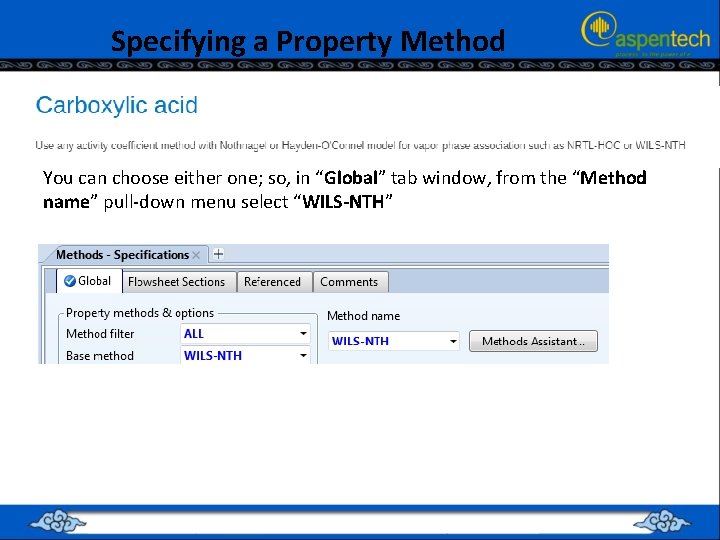
Specifying a Property Method • Clicking on the “Methods Assistant…” button, will invoke the “Property Method Selection Assistant” wizard • Select “Specify process type”; followed by selecting the type of process to be “Chemical”; and finally clicking “Carboxylic acids” as a subset of Chemical” processes. • This will guide you to either “NRTL-HOC” or “WILS-NTH”

Specifying a Property Method You can choose either one; so, in “Global” tab window, from the “Method name” pull-down menu select “WILS-NTH”

Binary Interaction Parameter Ø Under “Methods” | “Parameters” | “Binary Interaction” | “WILSON-1” sheet be sure that the “Estimate missing parameters by UNIFAC” option is checked. Ø Click “Reset” followed by “Next” button to run the simulation and assure that properties analysis completed successfully. Ø Control Panel” shows a warning as follows: Ø Such a warning can be ignored given the fact that the pairwise interaction parameters are already calculated by UNIFAC method and are shown in “Parameters” |“Binary Interaction” | “WILSON-1” sheet with the source as R-PCES. Ø To remove this persisting warning, deselect the option “Estimate missing parameters by UNIFAC”.

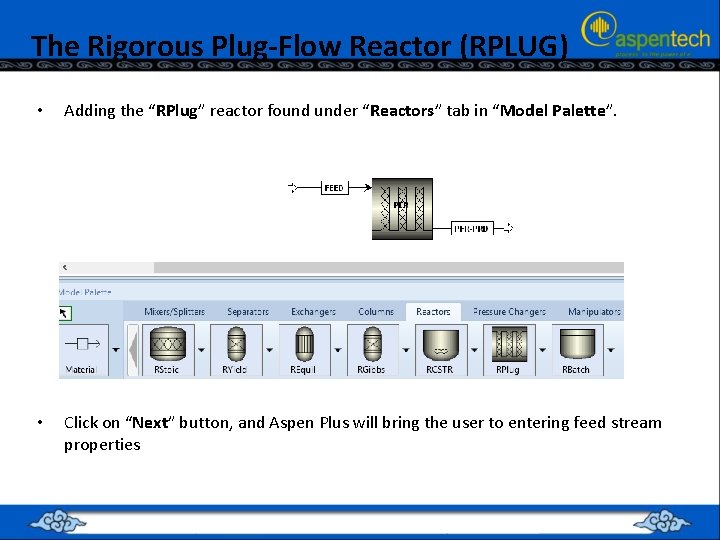
The Rigorous Plug-Flow Reactor (RPLUG) • Adding the “RPlug” reactor found under “Reactors” tab in “Model Palette”. • Click on “Next” button, and Aspen Plus will bring the user to entering feed stream properties

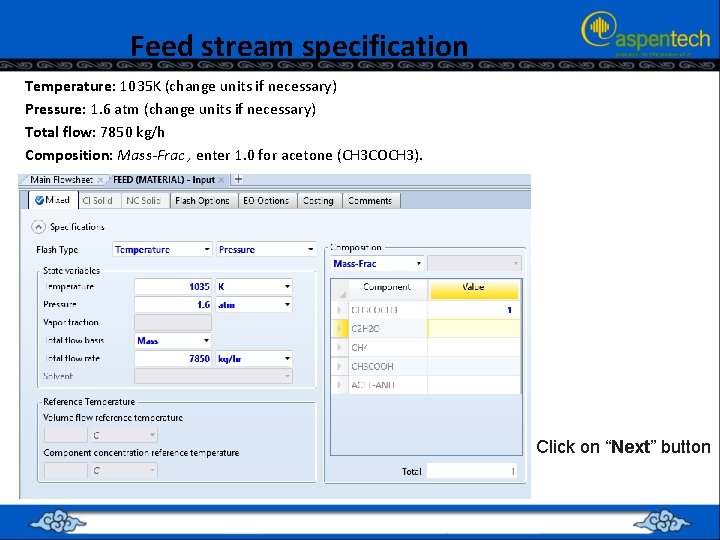
Feed stream specification Temperature: 1035 K (change units if necessary) Pressure: 1. 6 atm (change units if necessary) Total flow: 7850 kg/h Composition: Mass-Frac , enter 1. 0 for acetone (CH 3 COCH 3). Click on “Next” button

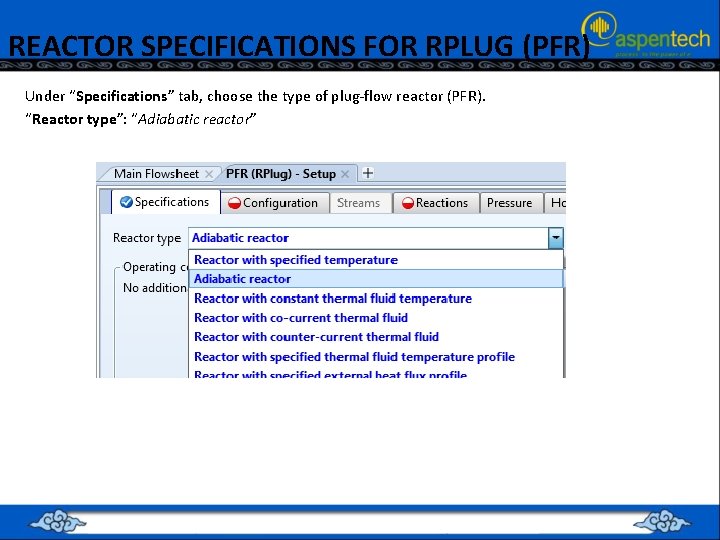
REACTOR SPECIFICATIONS FOR RPLUG (PFR) Under “Specifications” tab, choose the type of plug-flow reactor (PFR). “Reactor type”: “Adiabatic reactor”

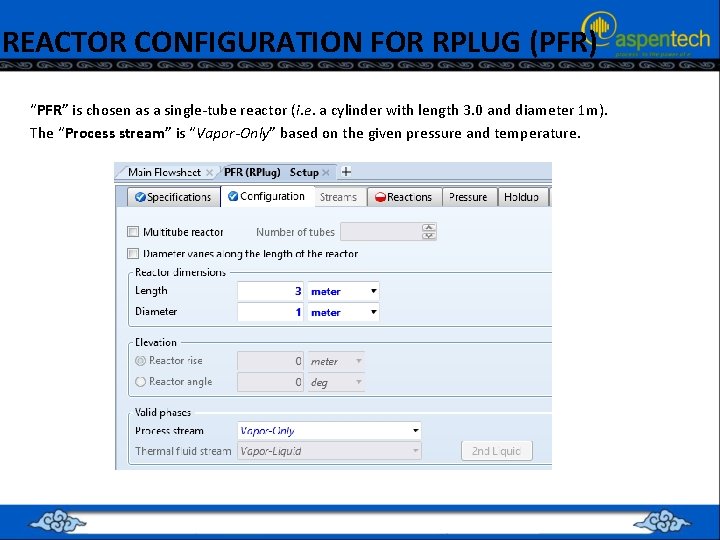
REACTOR CONFIGURATION FOR RPLUG (PFR) “PFR” is chosen as a single-tube reactor (i. e. a cylinder with length 3. 0 and diameter 1 m). The “Process stream” is “Vapor-Only” based on the given pressure and temperature.

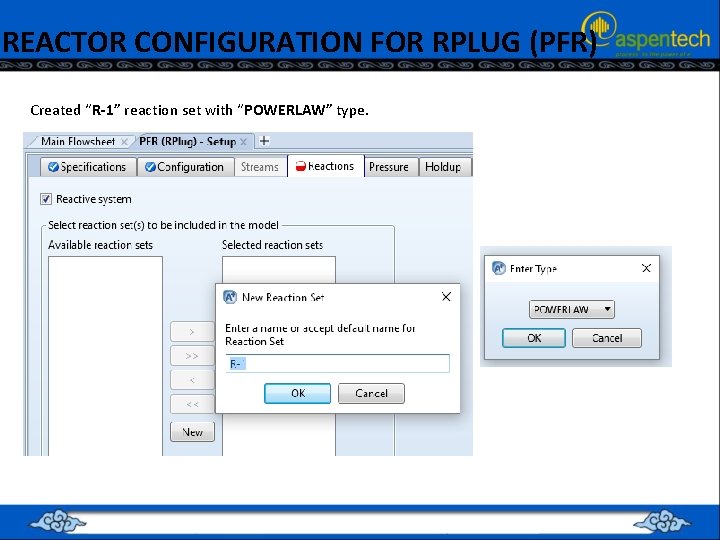
REACTOR CONFIGURATION FOR RPLUG (PFR) Created “R-1” reaction set with “POWERLAW” type.

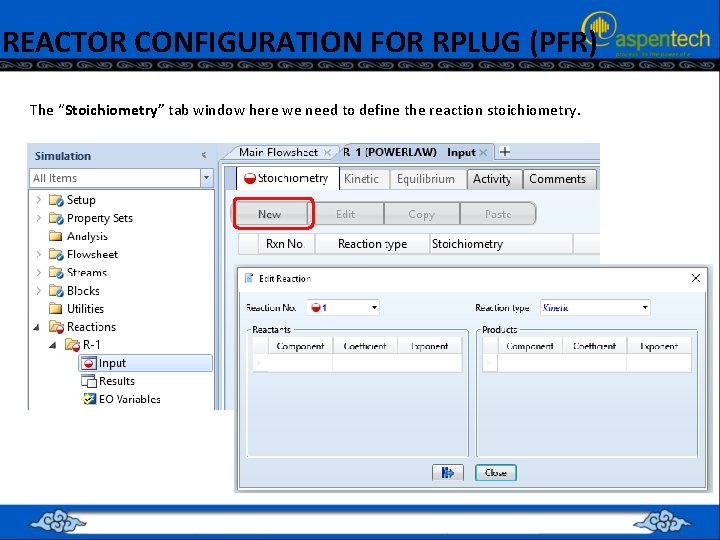
REACTOR CONFIGURATION FOR RPLUG (PFR) The “Stoichiometry” tab window here we need to define the reaction stoichiometry.

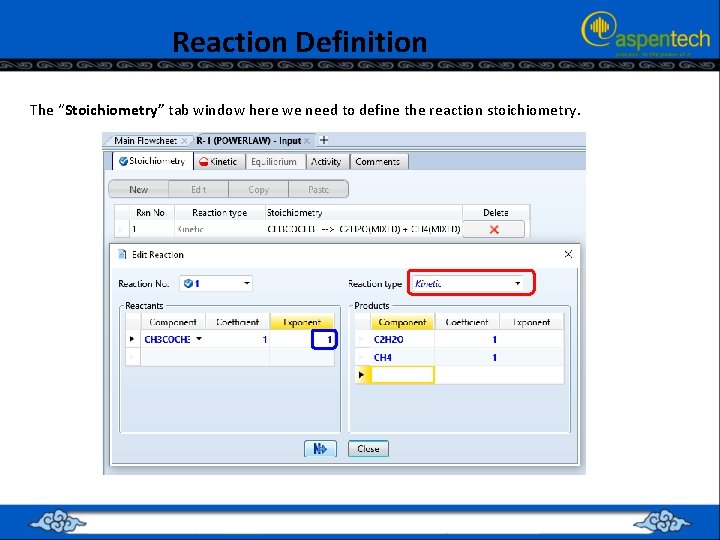
Reaction Definition The “Stoichiometry” tab window here we need to define the reaction stoichiometry.

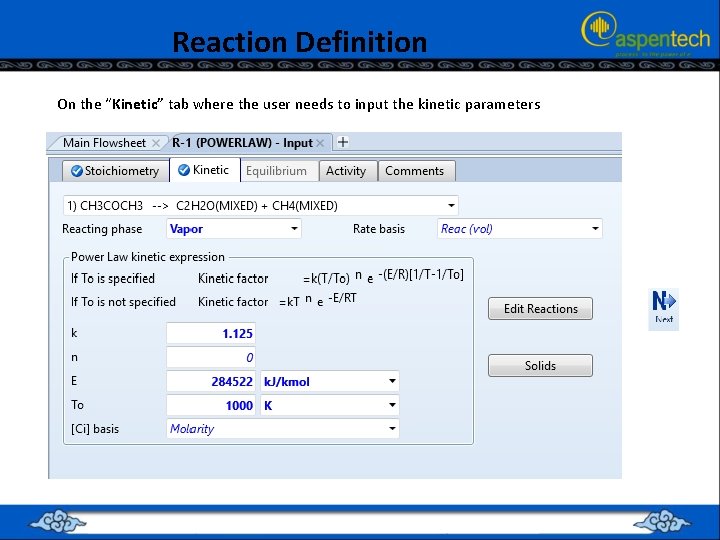
Reaction Definition On the “Kinetic” tab where the user needs to input the kinetic parameters

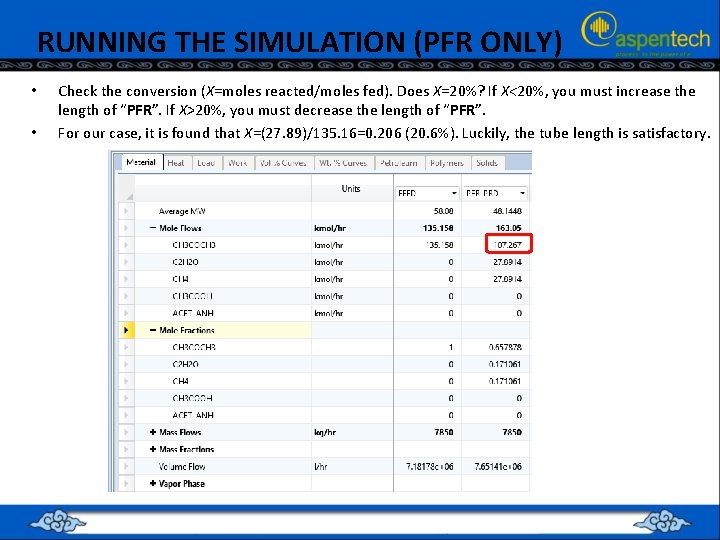
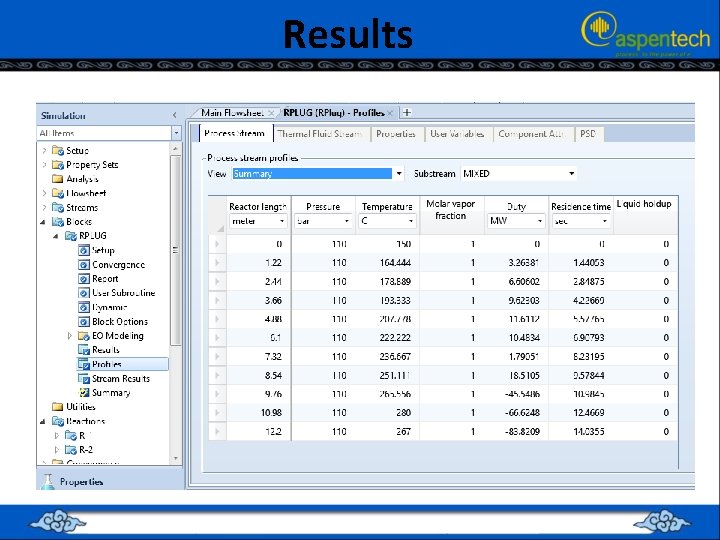
RUNNING THE SIMULATION (PFR ONLY) • • Check the conversion (X=moles reacted/moles fed). Does X=20%? If X<20%, you must increase the length of “PFR”. If X>20%, you must decrease the length of “PFR”. For our case, it is found that X=(27. 89)/135. 16=0. 206 (20. 6%). Luckily, the tube length is satisfactory.

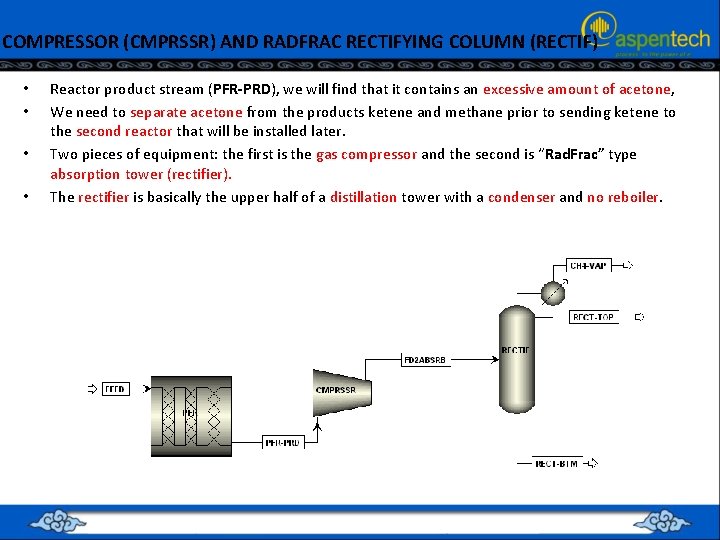
COMPRESSOR (CMPRSSR) AND RADFRAC RECTIFYING COLUMN (RECTIF) • • Reactor product stream (PFR-PRD), we will find that it contains an excessive amount of acetone, We need to separate acetone from the products ketene and methane prior to sending ketene to the second reactor that will be installed later. Two pieces of equipment: the first is the gas compressor and the second is “Rad. Frac” type absorption tower (rectifier). The rectifier is basically the upper half of a distillation tower with a condenser and no reboiler.

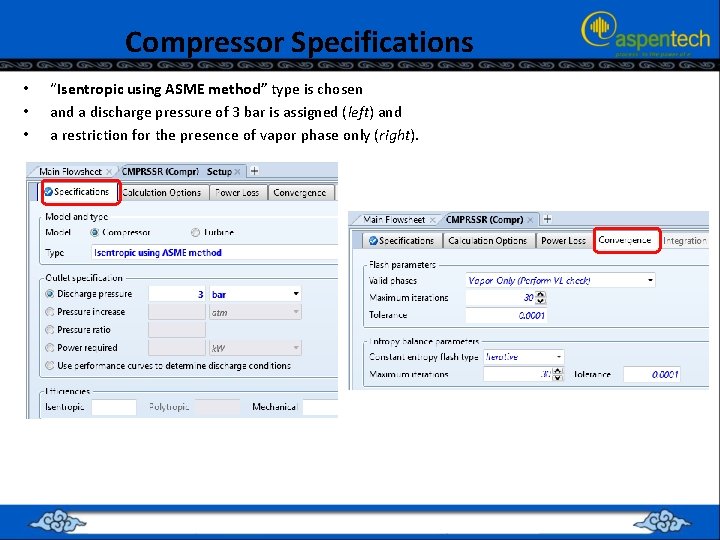
Compressor Specifications • • • “Isentropic using ASME method” type is chosen and a discharge pressure of 3 bar is assigned (left) and a restriction for the presence of vapor phase only (right).

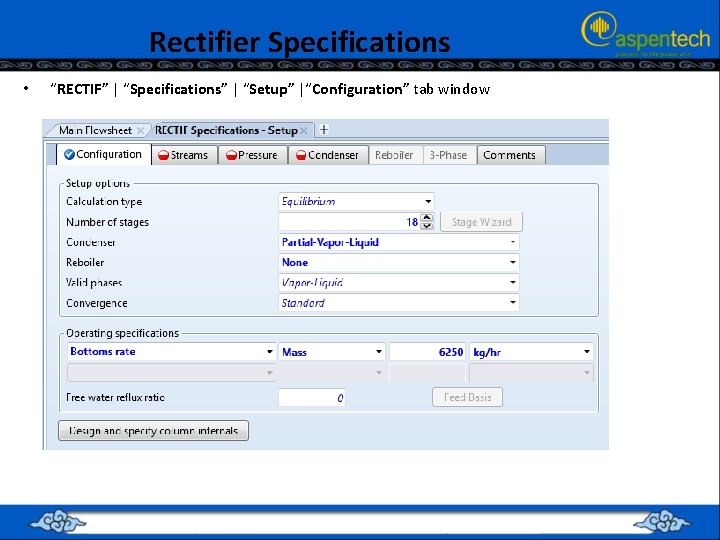
Rectifier Specifications • “RECTIF” | “Specifications” | “Setup” |“Configuration” tab window

Rectifier Specifications • • • Notice that the location of the feed tray is way down at the bottom of the rectifier. This makes sense as we need not have a reboiler; the feed stream will be available as the vapor phase throughout the entire rectifying column.

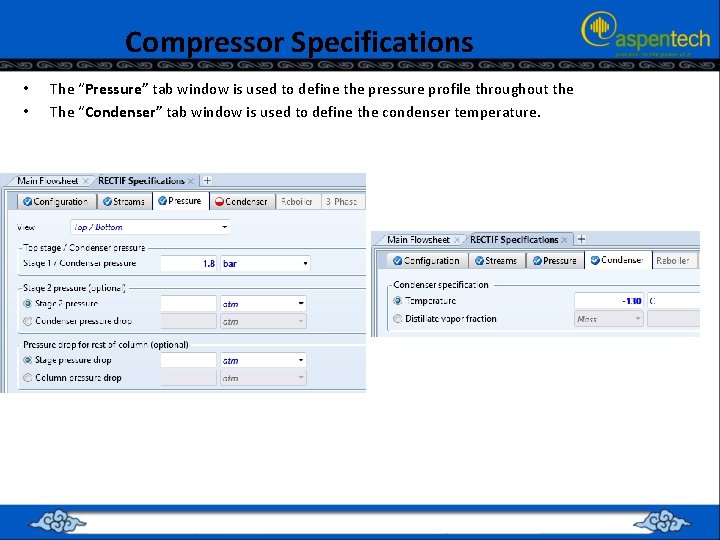
Compressor Specifications • • The “Pressure” tab window is used to define the pressure profile throughout the The “Condenser” tab window is used to define the condenser temperature.

RUNNING THE SIMULATION (PFR + CMPRSSR + RECTIF) • Click on “Reset” followed by “Next” and by “OK” button to allow Aspen Plus to do the calculations.

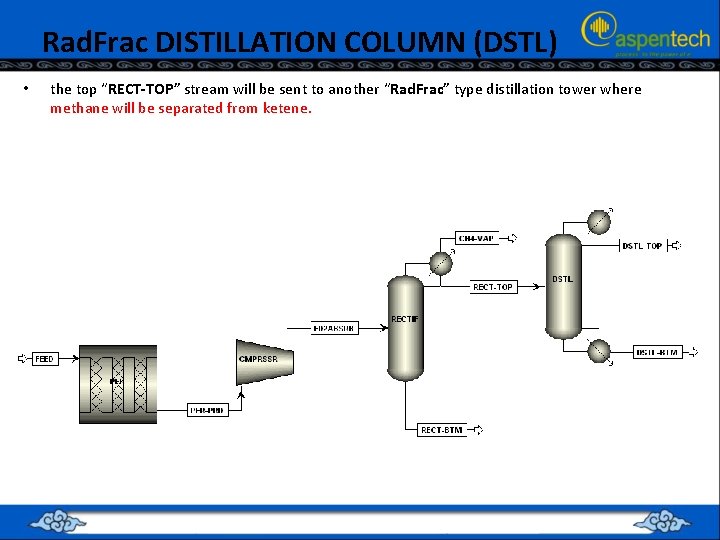
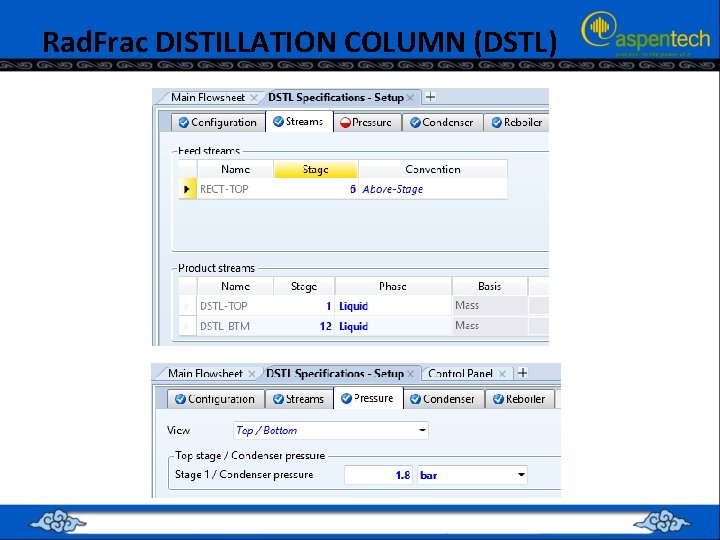
Rad. Frac DISTILLATION COLUMN (DSTL) • the top “RECT-TOP” stream will be sent to another “Rad. Frac” type distillation tower where methane will be separated from ketene.

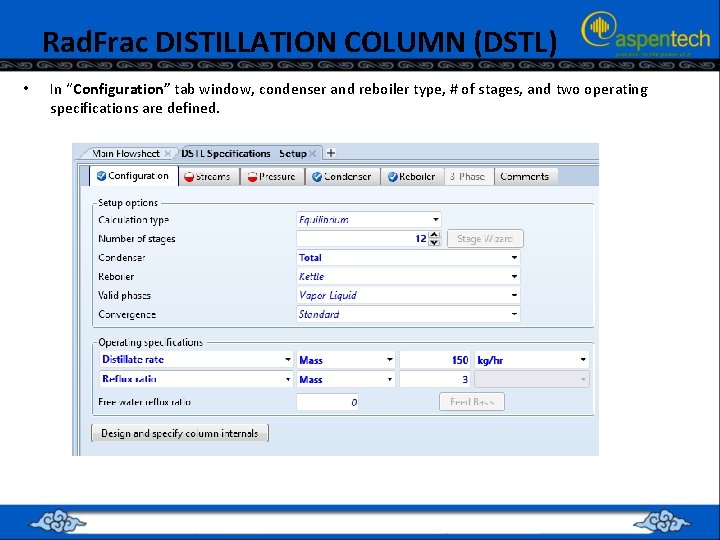
Rad. Frac DISTILLATION COLUMN (DSTL) • In “Configuration” tab window, condenser and reboiler type, # of stages, and two operating specifications are defined.

Rad. Frac DISTILLATION COLUMN (DSTL)

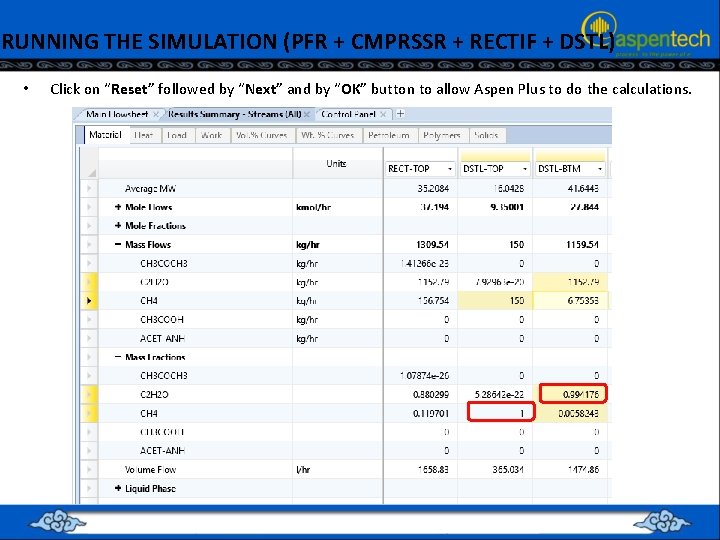
RUNNING THE SIMULATION (PFR + CMPRSSR + RECTIF + DSTL) • Click on “Reset” followed by “Next” and by “OK” button to allow Aspen Plus to do the calculations.

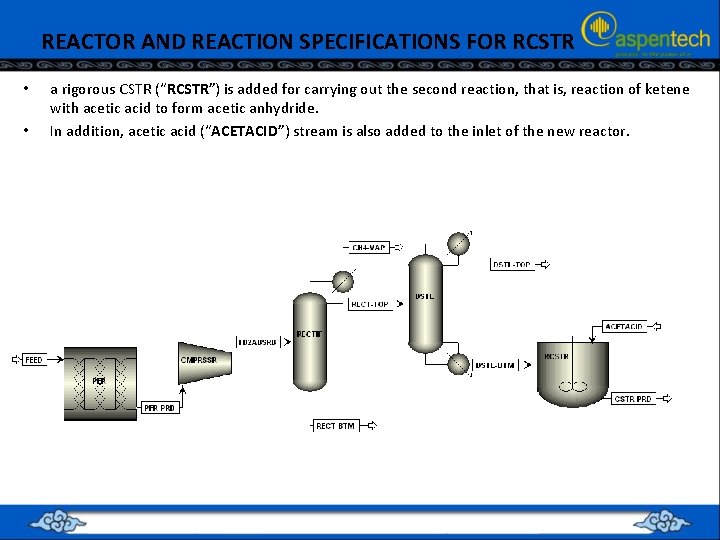
REACTOR AND REACTION SPECIFICATIONS FOR RCSTR • • a rigorous CSTR (“RCSTR”) is added for carrying out the second reaction, that is, reaction of ketene with acetic acid to form acetic anhydride. In addition, acetic acid (“ACETACID”) stream is also added to the inlet of the new reactor.

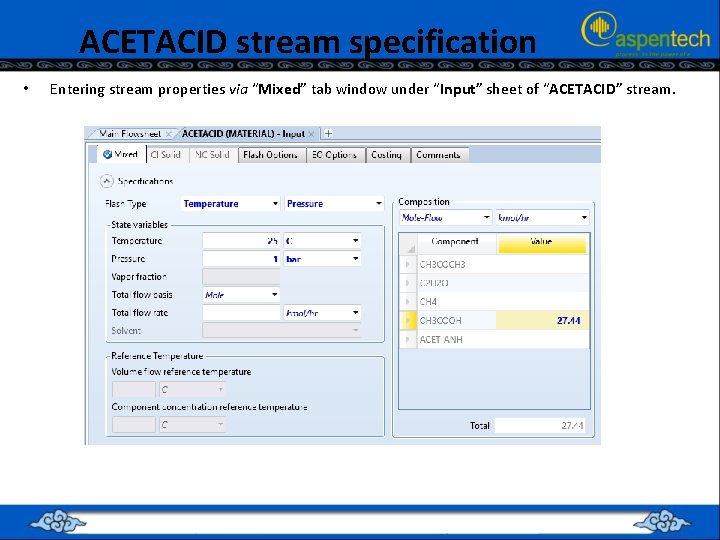
ACETACID stream specification • Entering stream properties via “Mixed” tab window under “Input” sheet of “ACETACID” stream.

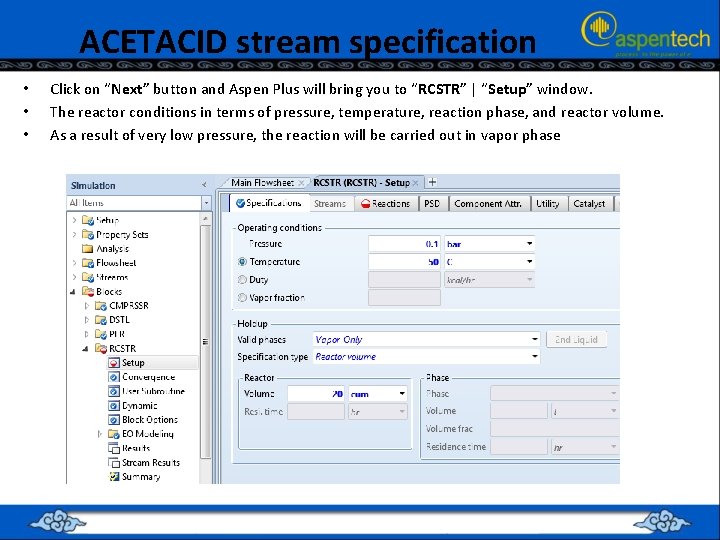
ACETACID stream specification • • • Click on “Next” button and Aspen Plus will bring you to “RCSTR” | “Setup” window. The reactor conditions in terms of pressure, temperature, reaction phase, and reactor volume. As a result of very low pressure, the reaction will be carried out in vapor phase

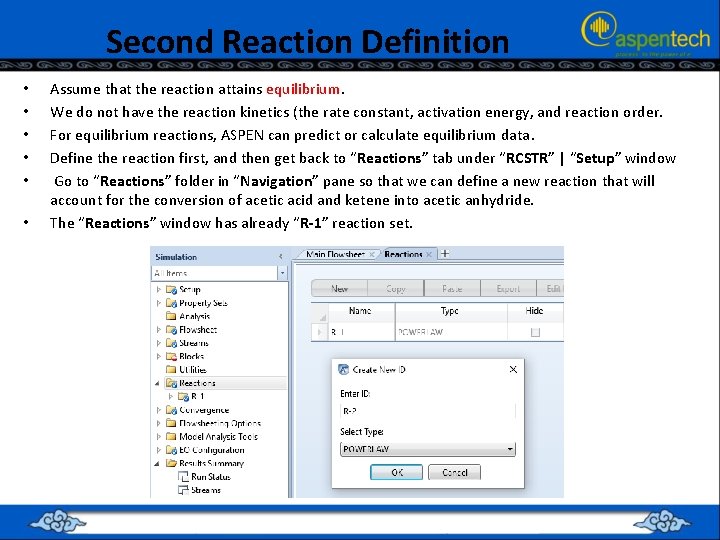
Second Reaction Definition • • • Assume that the reaction attains equilibrium. We do not have the reaction kinetics (the rate constant, activation energy, and reaction order. For equilibrium reactions, ASPEN can predict or calculate equilibrium data. Define the reaction first, and then get back to “Reactions” tab under “RCSTR” | “Setup” window Go to “Reactions” folder in “Navigation” pane so that we can define a new reaction that will account for the conversion of acetic acid and ketene into acetic anhydride. The “Reactions” window has already “R-1” reaction set.

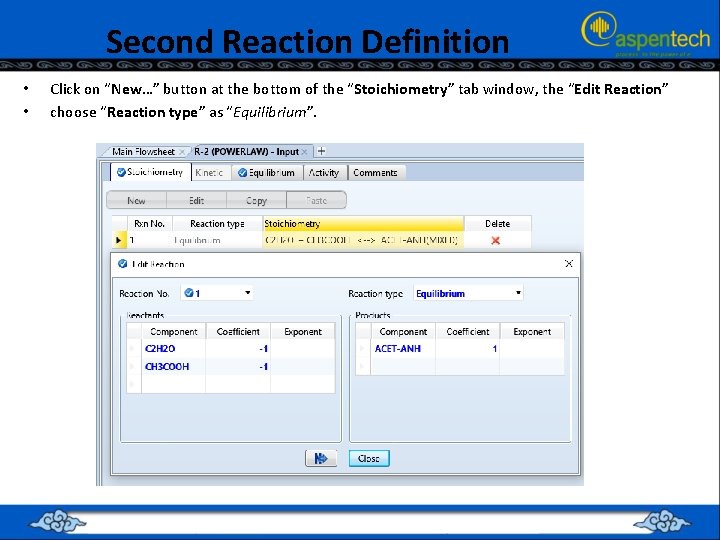
Second Reaction Definition • • Click on “New…” button at the bottom of the “Stoichiometry” tab window, the “Edit Reaction” choose “Reaction type” as “Equilibrium”.

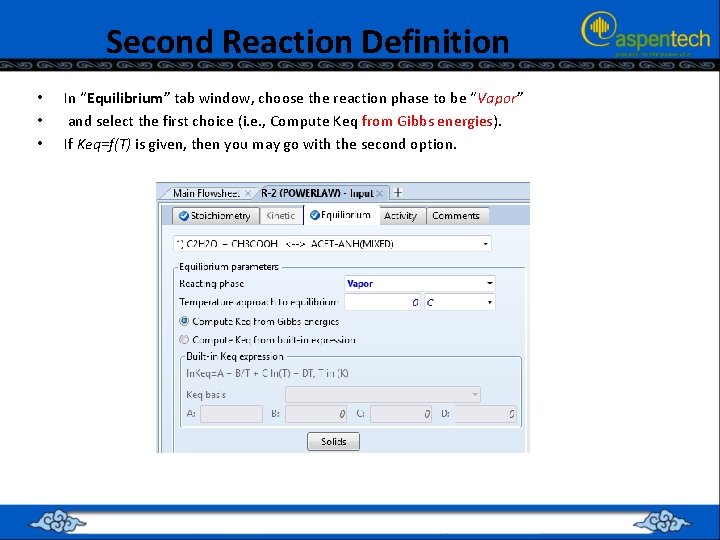
Second Reaction Definition • • • In “Equilibrium” tab window, choose the reaction phase to be “Vapor” and select the first choice (i. e. , Compute Keq from Gibbs energies). If Keq=f(T) is given, then you may go with the second option.

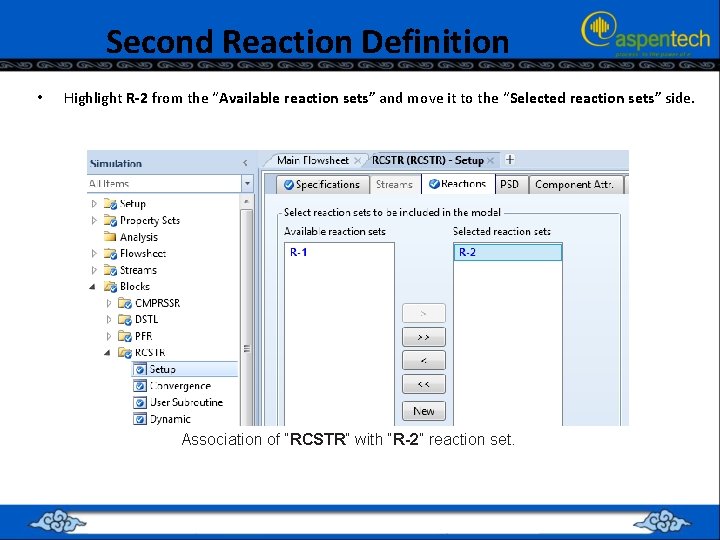
Second Reaction Definition • Highlight R-2 from the “Available reaction sets” and move it to the “Selected reaction sets” side. Association of “RCSTR” with “R 2” reaction set.

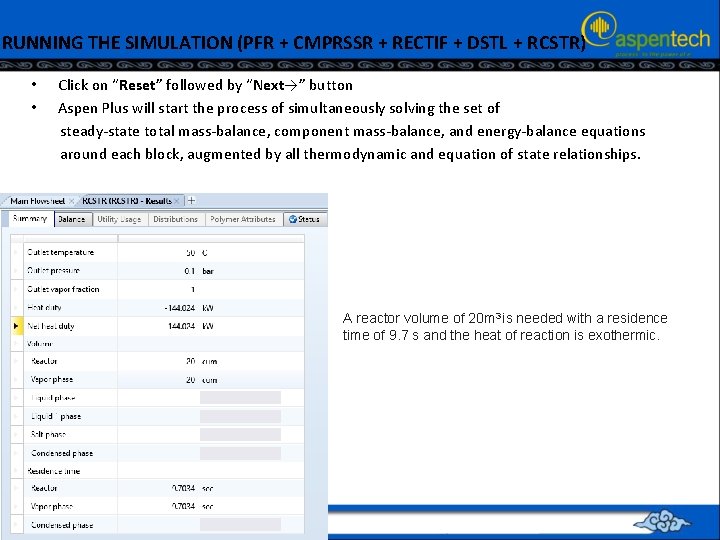
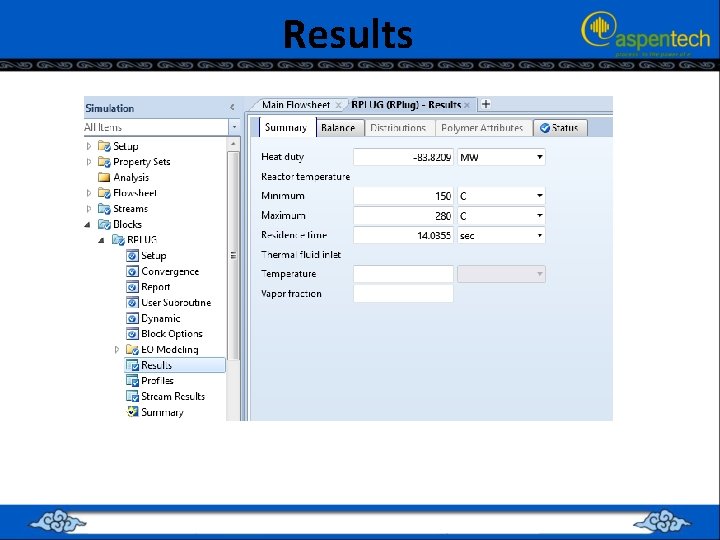
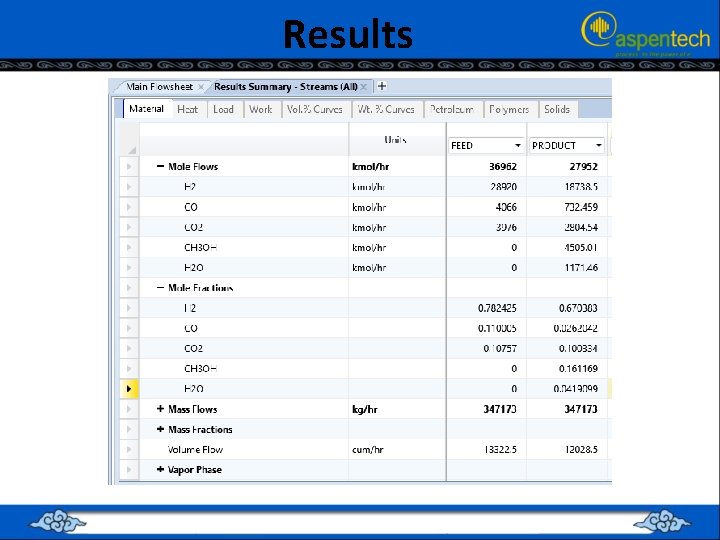
RUNNING THE SIMULATION (PFR + CMPRSSR + RECTIF + DSTL + RCSTR) • • Click on “Reset” followed by “Next→” button Aspen Plus will start the process of simultaneously solving the set of steady-state total mass-balance, component mass-balance, and energy-balance equations around each block, augmented by all thermodynamic and equation of state relationships. A reactor volume of 20 m 3 is needed with a residence time of 9. 7 s and the heat of reaction is exothermic.

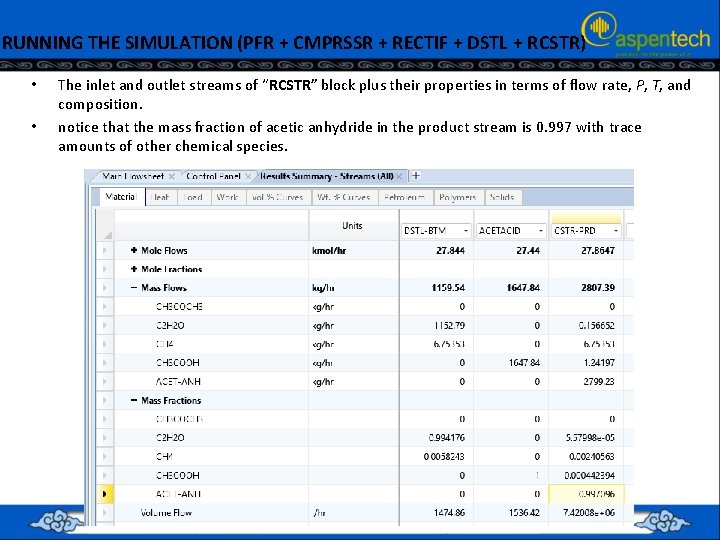
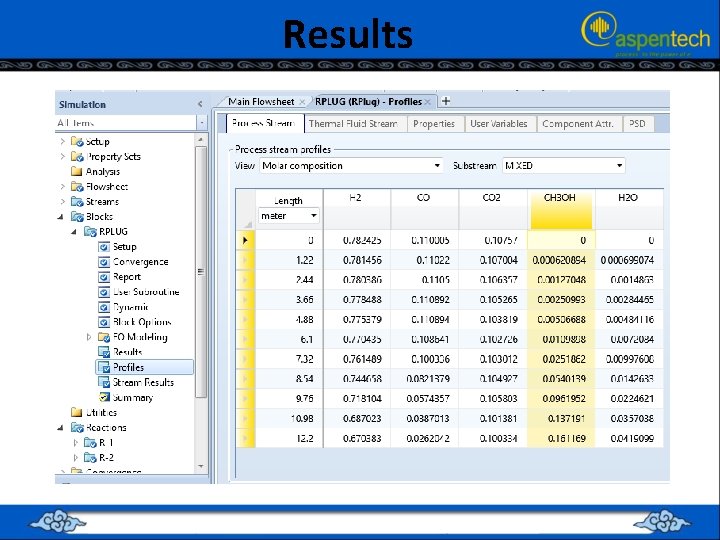
RUNNING THE SIMULATION (PFR + CMPRSSR + RECTIF + DSTL + RCSTR) • • The inlet and outlet streams of “RCSTR” block plus their properties in terms of flow rate, P, T, and composition. notice that the mass fraction of acetic anhydride in the product stream is 0. 997 with trace amounts of other chemical species.

REACTORS WITH COMPLEX (NON-CONVENTIONAL) REACTION KINETIC FORMS Using Aspen Plus® V 9. 0

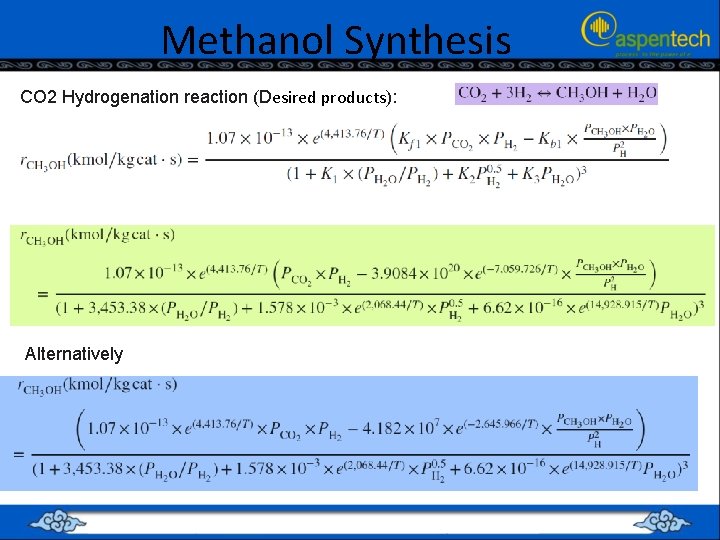
Methanol Synthesis CO 2 Hydrogenation reaction (Desired products): Alternatively

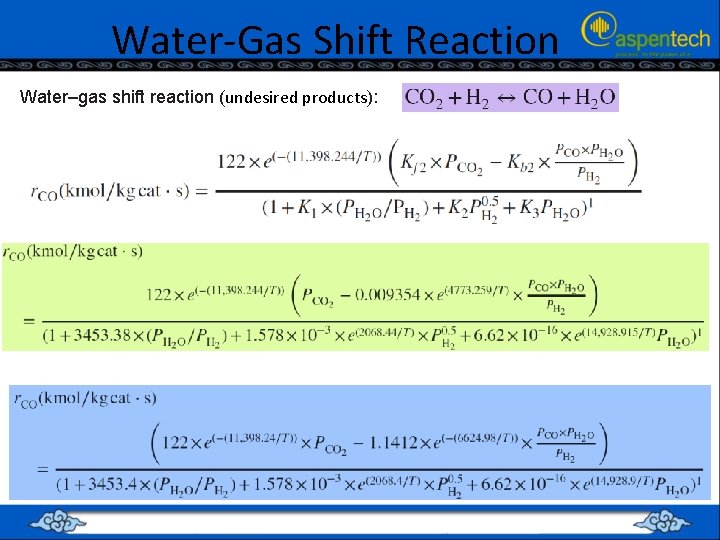
Water-Gas Shift Reaction Water–gas shift reaction (undesired products):

Methanol Synthesis • Both reactions are solid-catalyzed exothermic reactions • Their kinetic forms, obey what is called Langmuir–Hinshelwood–Hougen– Watson (LHHW) form. • It was found that using light green equations ended up with either simulation error or non-sense results for selecting a different basis for feed stream composition. • On the other hand, using light blue equations ended up with reasonable results for selecting a different basis for feed stream composition. • Aspen Plus expects from the user to express the kinetic factor with a positive activation energy. • Hence, we will use light blue equations, where the kinetic factor is merged into the driving force expression, ending up with a kinetic factor equal to unity.

Langmuir–Hinshelwood–Hougen–Watson (LHHW) • The general LHHW expression is: • Kinetic factor: • Driving force expression: • Adsorption term:

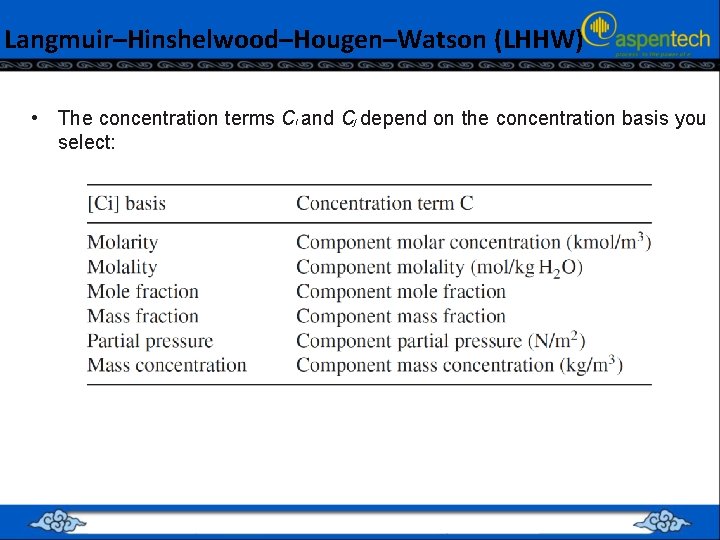
Langmuir–Hinshelwood–Hougen–Watson (LHHW) • The concentration terms Ci and Cj depend on the concentration basis you select:

THE PROPERTY METHOD: “SRK” • Choose “Chemicals with Metric Units” template to create a steady-state flowsheet • • Since methanol production takes place at a pressure greater than 10 bar, then using the Property Method Selection Assistant, it is recommended to use an equation of state, such as “SR-POLAR”, “SRK”, and “PSRK”. “SRK” was selected. in “Methods” | “Specifications” | “Global” tab window set the “Free-water method” to “STEAMNBS”

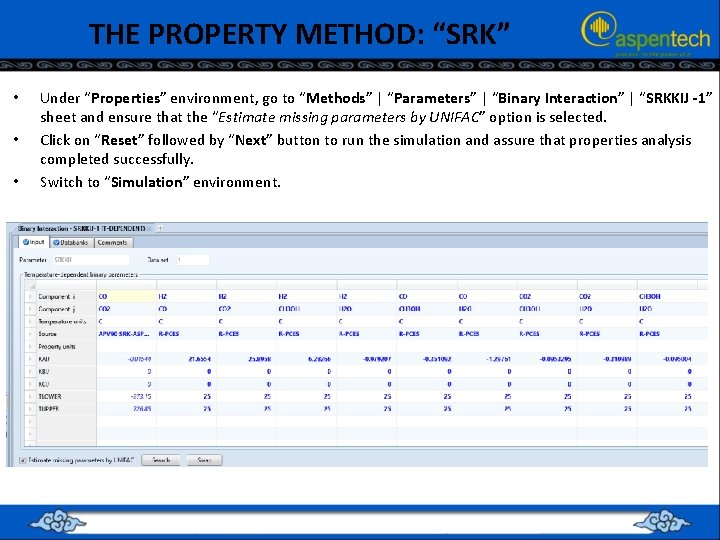
THE PROPERTY METHOD: “SRK” • • • Under “Properties” environment, go to “Methods” | “Parameters” | “Binary Interaction” | “SRKKIJ -1” sheet and ensure that the “Estimate missing parameters by UNIFAC” option is selected. Click on “Reset” followed by “Next” button to run the simulation and assure that properties analysis completed successfully. Switch to “Simulation” environment.

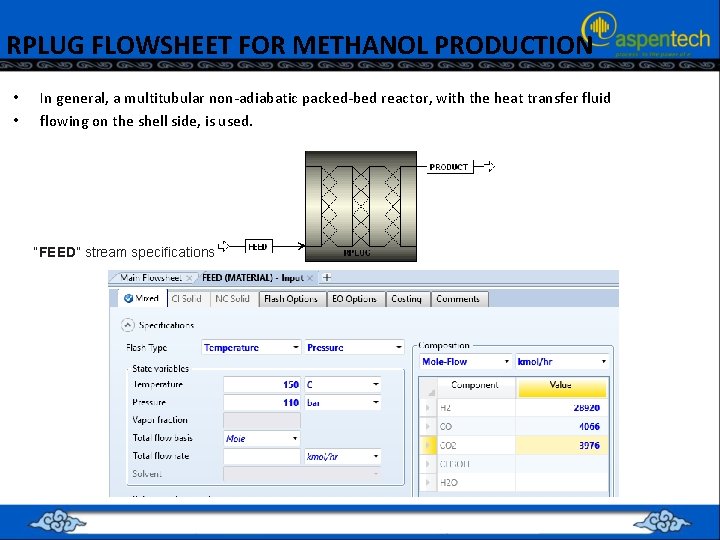
RPLUG FLOWSHEET FOR METHANOL PRODUCTION • • In general, a multitubular non-adiabatic packed-bed reactor, with the heat transfer fluid flowing on the shell side, is used. “FEED” stream specifications

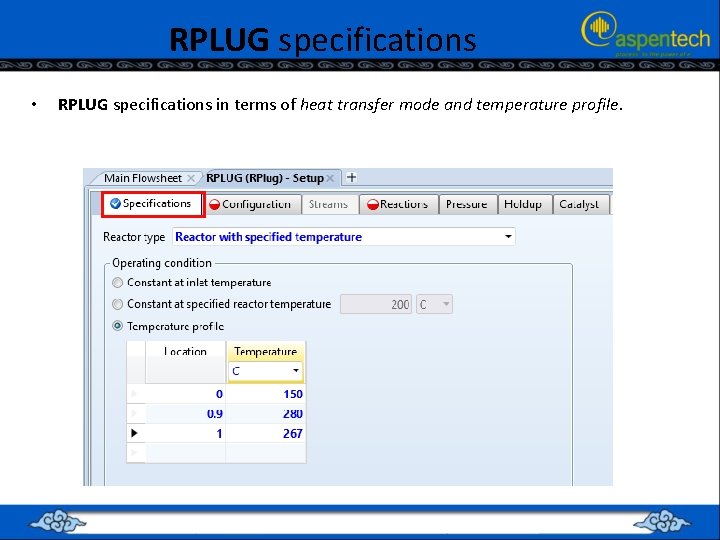
RPLUG specifications • RPLUG specifications in terms of heat transfer mode and temperature profile.

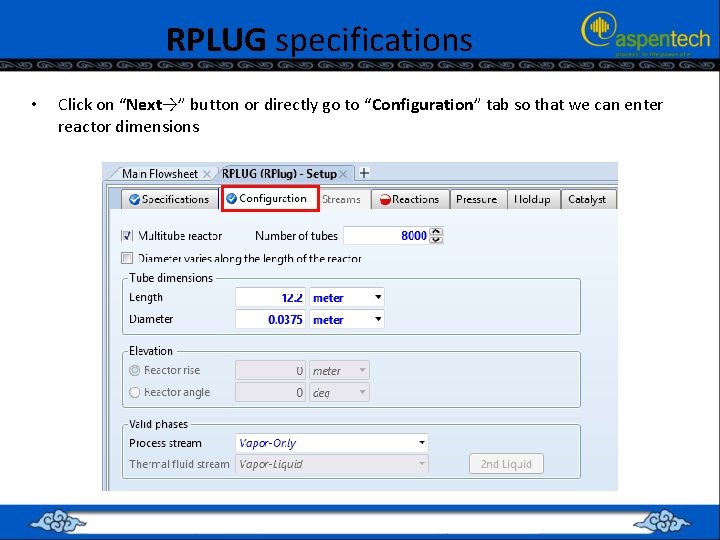
RPLUG specifications • Click on “Next→” button or directly go to “Configuration” tab so that we can enter reactor dimensions

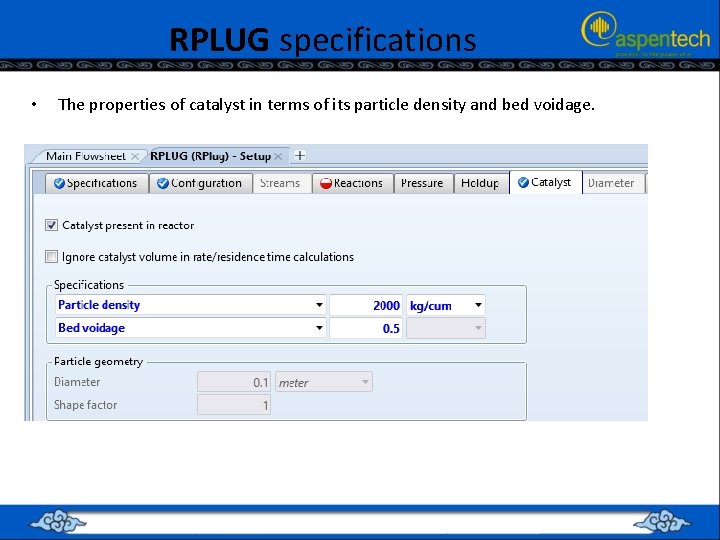
RPLUG specifications • The properties of catalyst in terms of its particle density and bed voidage.

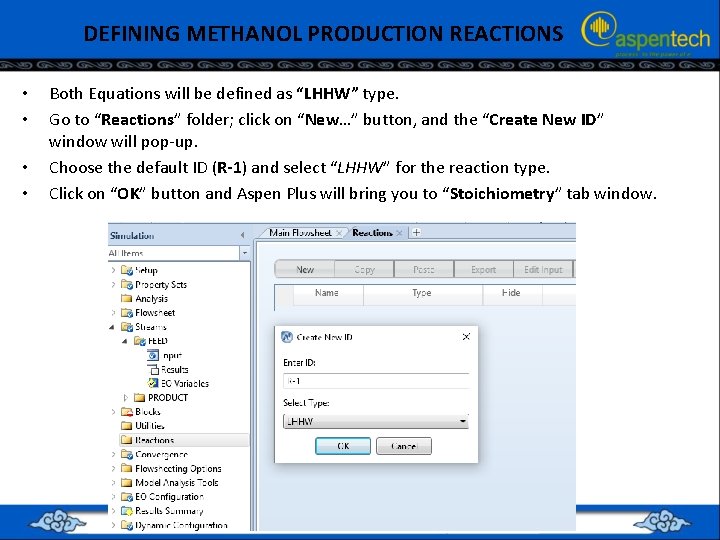
DEFINING METHANOL PRODUCTION REACTIONS • • Both Equations will be defined as “LHHW” type. Go to “Reactions” folder; click on “New…” button, and the “Create New ID” window will pop-up. Choose the default ID (R-1) and select “LHHW” for the reaction type. Click on “OK” button and Aspen Plus will bring you to “Stoichiometry” tab window.

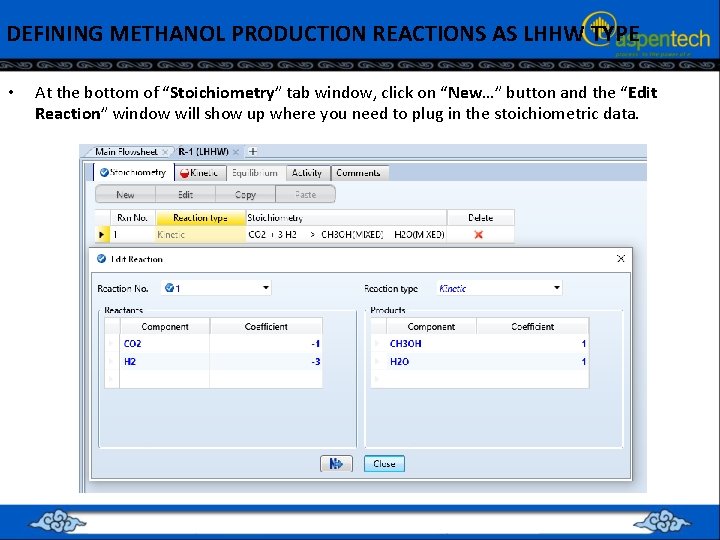
DEFINING METHANOL PRODUCTION REACTIONS AS LHHW TYPE • At the bottom of “Stoichiometry” tab window, click on “New…” button and the “Edit Reaction” window will show up where you need to plug in the stoichiometric data.

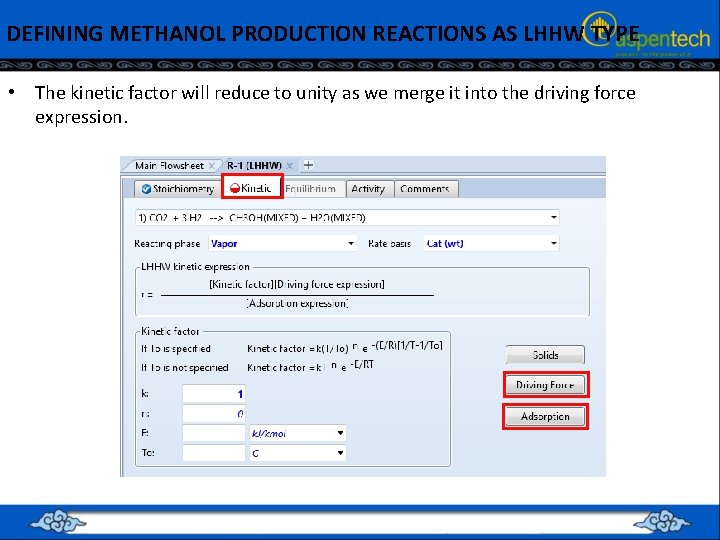
DEFINING METHANOL PRODUCTION REACTIONS AS LHHW TYPE • The kinetic factor will reduce to unity as we merge it into the driving force expression.

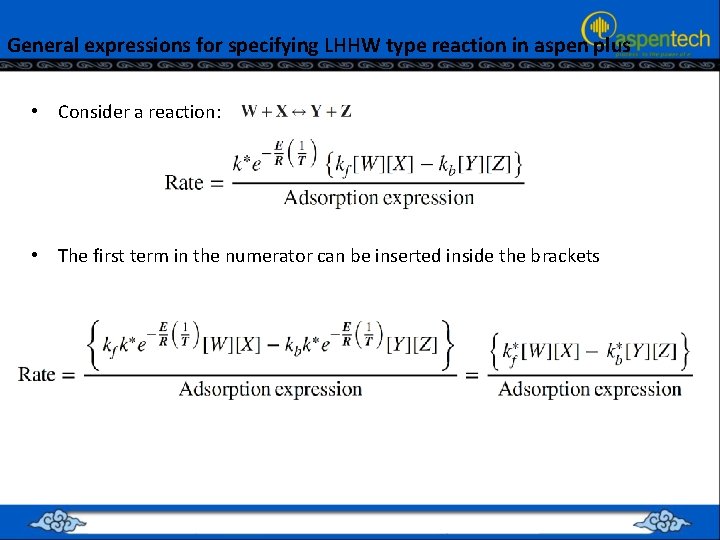
General expressions for specifying LHHW type reaction in aspen plus • Consider a reaction: • The first term in the numerator can be inserted inside the brackets

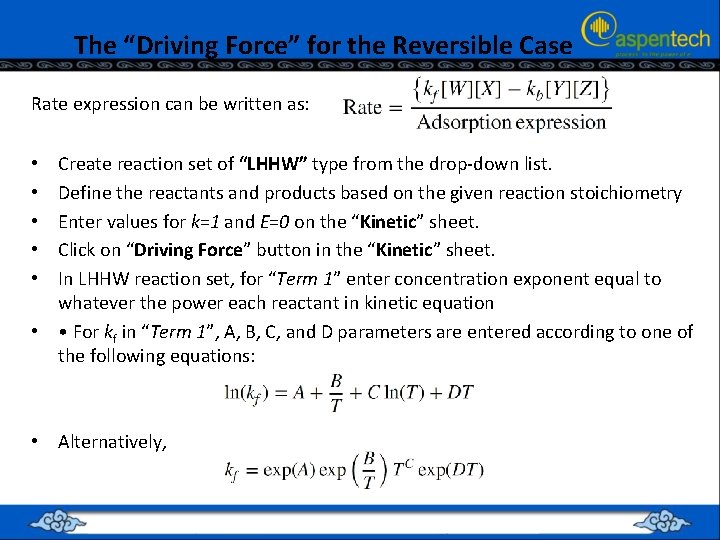
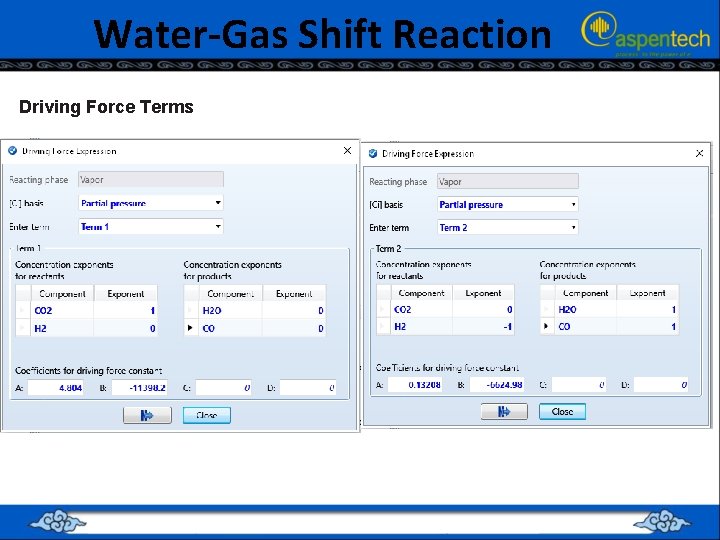
The “Driving Force” for the Reversible Case Rate expression can be written as: Create reaction set of “LHHW” type from the drop-down list. Define the reactants and products based on the given reaction stoichiometry Enter values for k=1 and E=0 on the “Kinetic” sheet. Click on “Driving Force” button in the “Kinetic” sheet. In LHHW reaction set, for “Term 1” enter concentration exponent equal to whatever the power each reactant in kinetic equation • • For kf in “Term 1”, A, B, C, and D parameters are entered according to one of the following equations: • • • Alternatively,

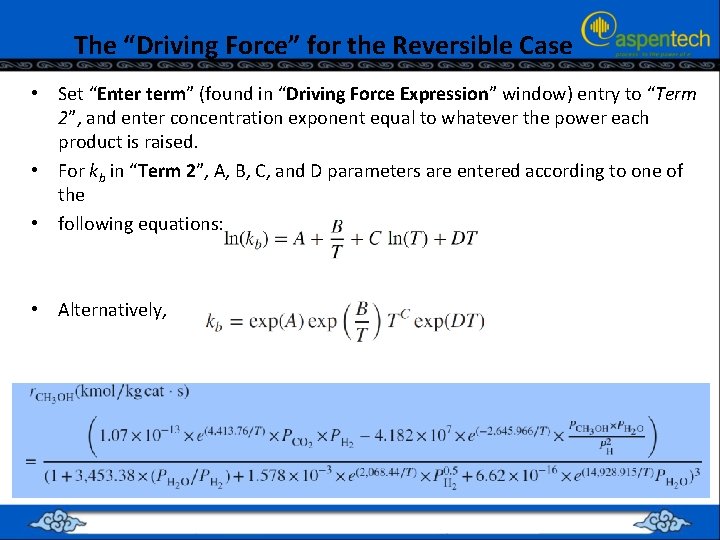
The “Driving Force” for the Reversible Case • Set “Enter term” (found in “Driving Force Expression” window) entry to “Term 2”, and enter concentration exponent equal to whatever the power each product is raised. • For kb in “Term 2”, A, B, C, and D parameters are entered according to one of the • following equations: • Alternatively,

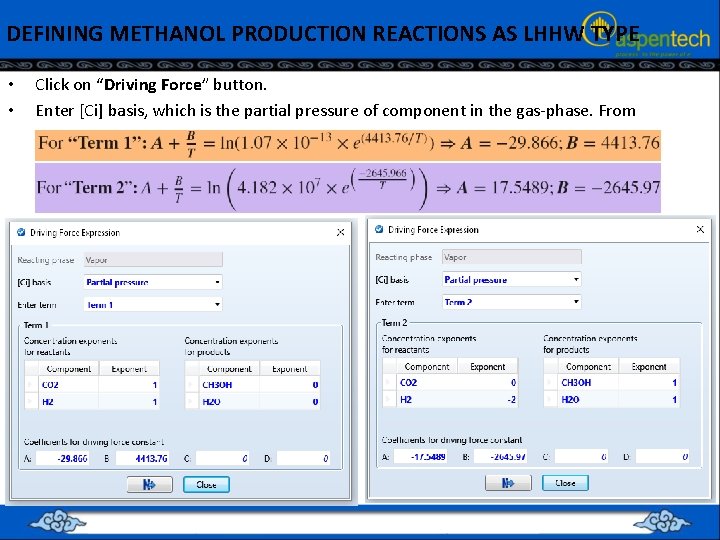
DEFINING METHANOL PRODUCTION REACTIONS AS LHHW TYPE • • Click on “Driving Force” button. Enter [Ci] basis, which is the partial pressure of component in the gas-phase. From

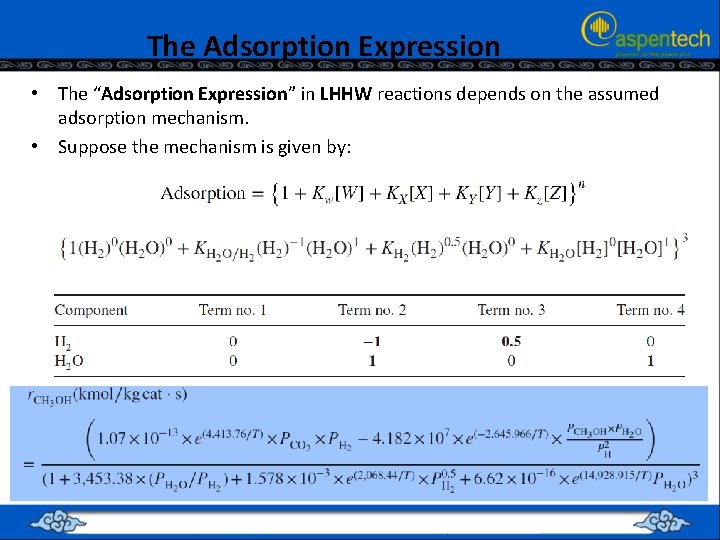
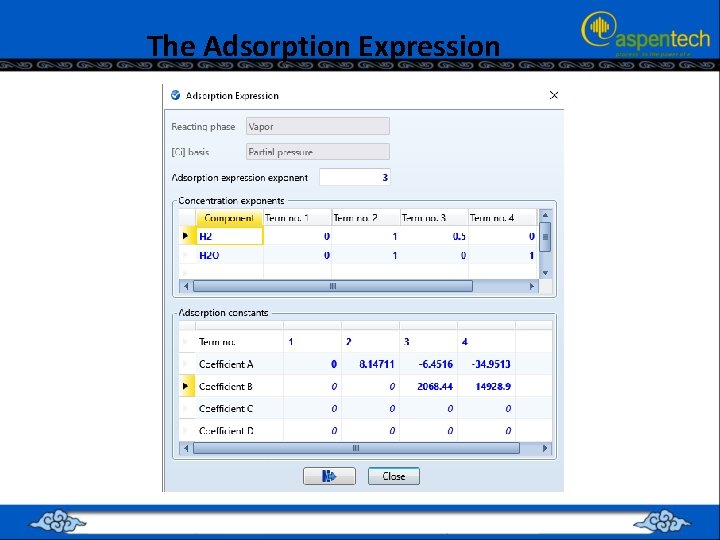
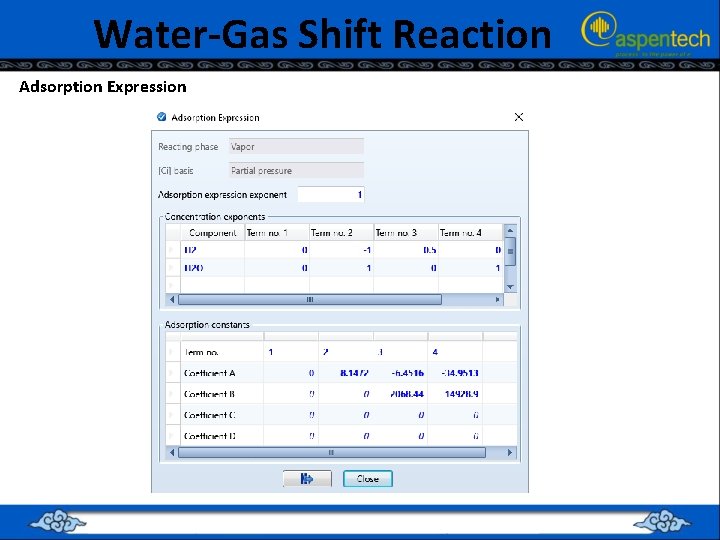
The Adsorption Expression • The “Adsorption Expression” in LHHW reactions depends on the assumed adsorption mechanism. • Suppose the mechanism is given by:

The Adsorption Expression

The Adsorption Expression

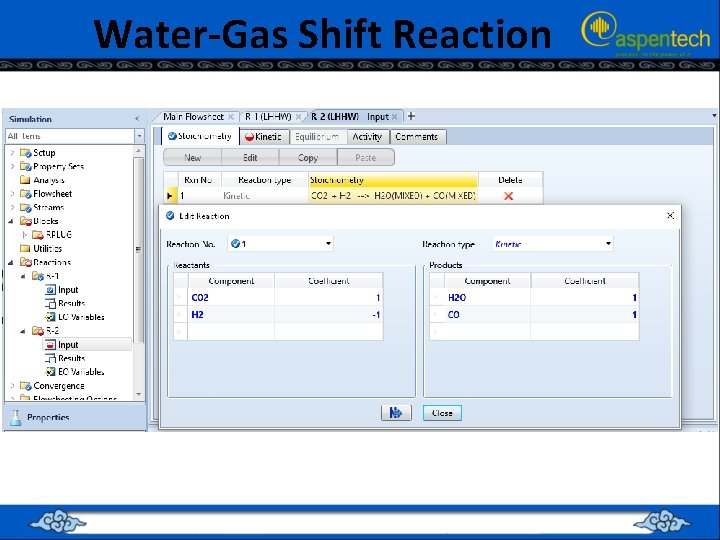
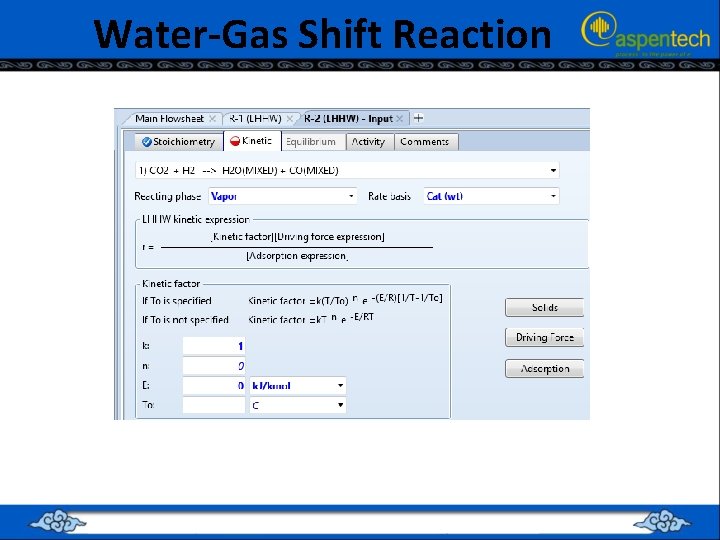
Water-Gas Shift Reaction

Water-Gas Shift Reaction

Water-Gas Shift Reaction Driving Force Terms

Water-Gas Shift Reaction Adsorption Expression

Results

Results

Results

Results


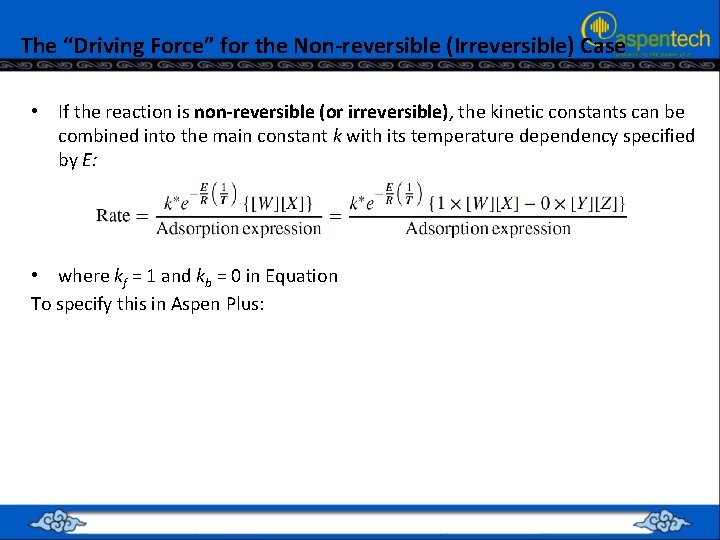
The “Driving Force” for the Non-reversible (Irreversible) Case • If the reaction is non-reversible (or irreversible), the kinetic constants can be combined into the main constant k with its temperature dependency specified by E: • where kf = 1 and kb = 0 in Equation To specify this in Aspen Plus:

The “Driving Force” for the Non-reversible (Irreversible) Case • Create a reaction set of “LHHW” type from the drop-down list. • Define the reactants and products based on the given reaction stoichiometry, Enter values for k and E on the “Kinetic” sheet. • Click on the “Driving Force” button in the “Kinetic” sheet. • In LHHW reaction set, for “Term 1” enter A=0 (i. e. , kf = 1 and A=ln(kf) = ln(1) = 0) and concentration exponent equal to whatever the power each reactant is raised to (e. g. , both [W] and [X] are each raised to power 1 in Equation 7. 13). • Set “Enter term” (found in “Driving Force Expression” window) entry to “Term • 2”, and enter a large negative value for A (i. e. , kb = 0 and A = ln(kb) = ln(0) = −∞). • NOTE: There is no need to enter exponents for products [Y] and [Z] as kb is already zero for the irreversible case.