COMPOUNDS WHAT IS A COMPOUND A compound is
- Slides: 13

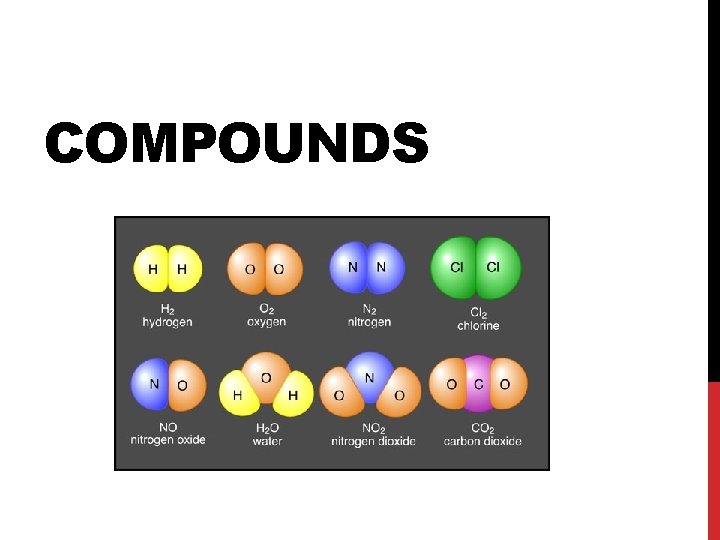
COMPOUNDS

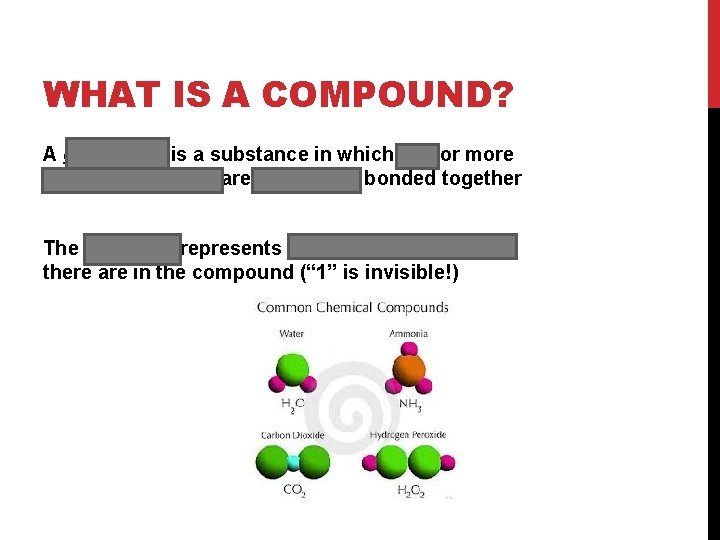
WHAT IS A COMPOUND? A compound is a substance in which two or more different elements are chemically bonded together The subscript represents how many of each atom there are in the compound (“ 1” is invisible!)

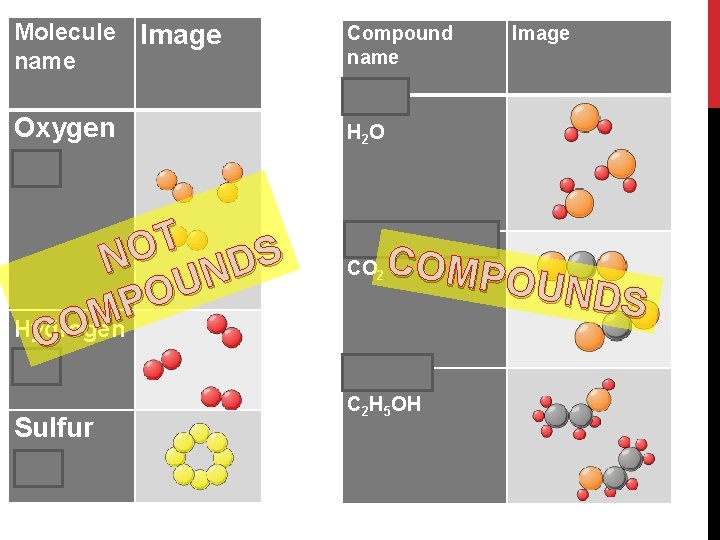
Molecule Image name Oxygen O 2 T NO NDS U O P M Hydrogen CO H 2 Sulfur S 8 Compound name Image Water H 2 O Carbon dioxide CO 2 COMP OUND S Alcohol C 2 H 5 OH

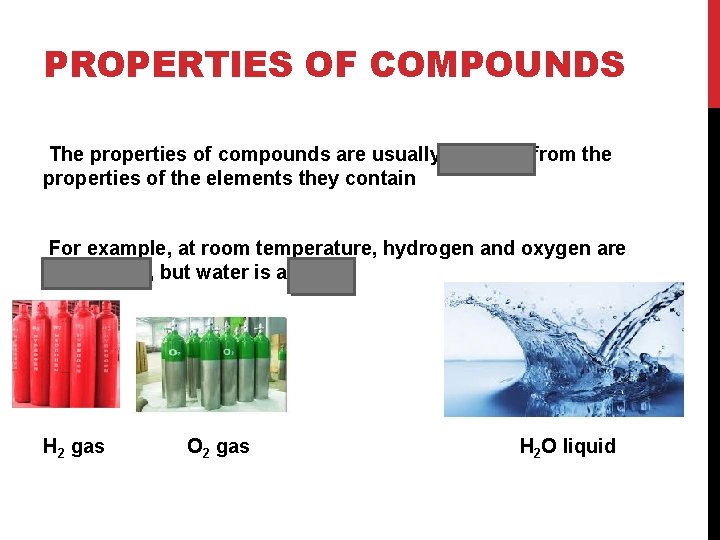
PROPERTIES OF COMPOUNDS The properties of compounds are usually different from the properties of the elements they contain For example, at room temperature, hydrogen and oxygen are both gases, but water is a liquid H 2 gas O 2 gas H 2 O liquid

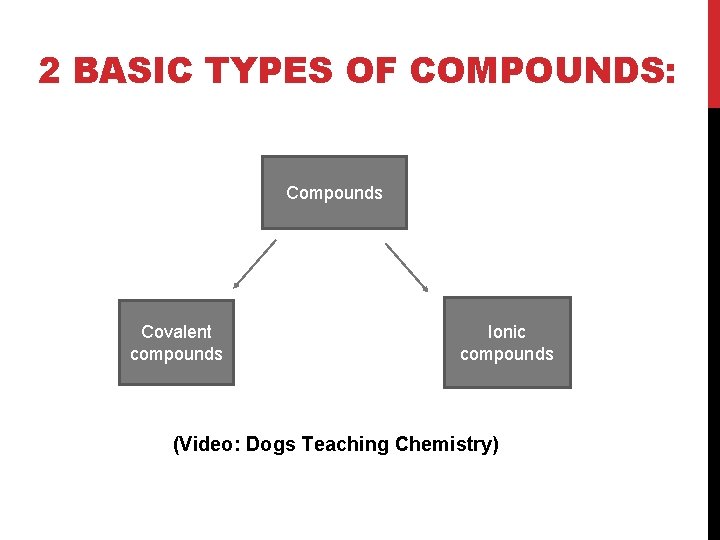
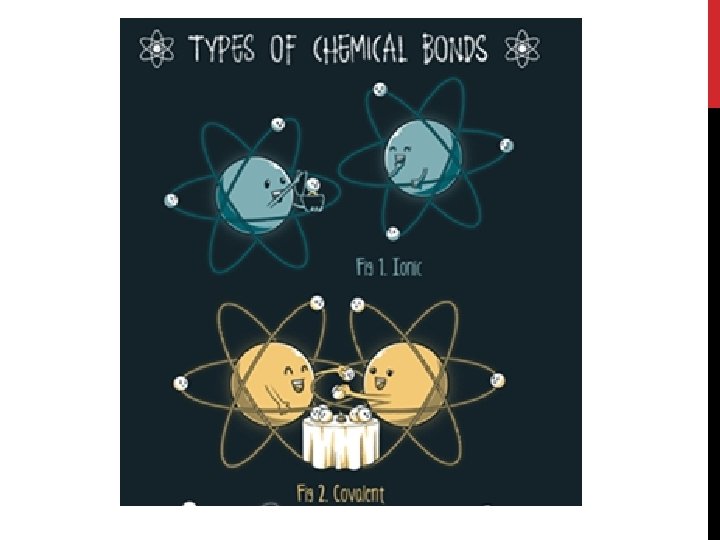
2 BASIC TYPES OF COMPOUNDS: Compounds Covalent compounds Ionic compounds (Video: Dogs Teaching Chemistry)

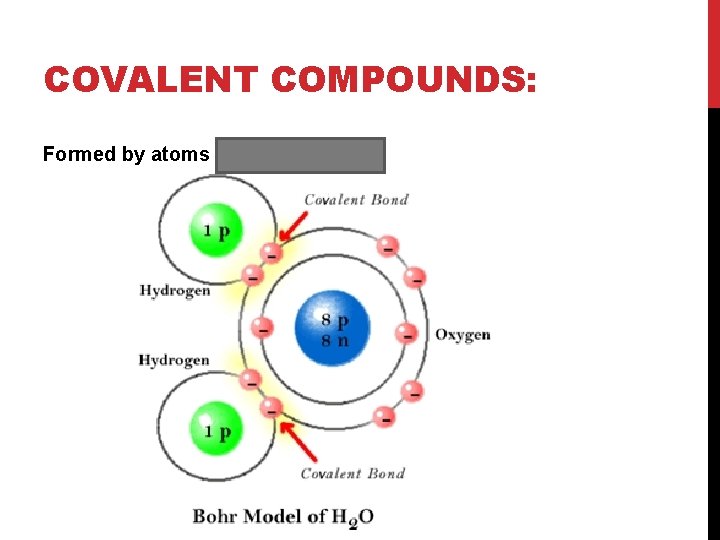
COVALENT COMPOUNDS: Formed by atoms sharing electrons

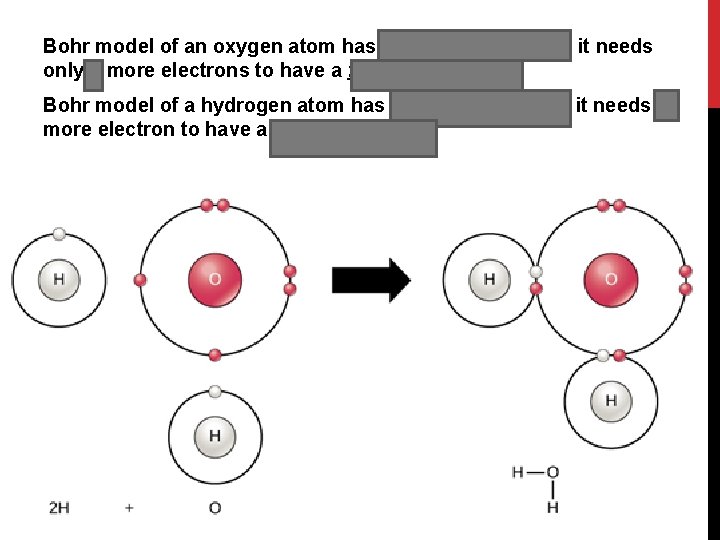
Bohr model of an oxygen atom has 6 valence electrons, it needs only 2 more electrons to have a full valence shell Bohr model of a hydrogen atom has 1 valence electron, it needs 1 more electron to have a full valence shell

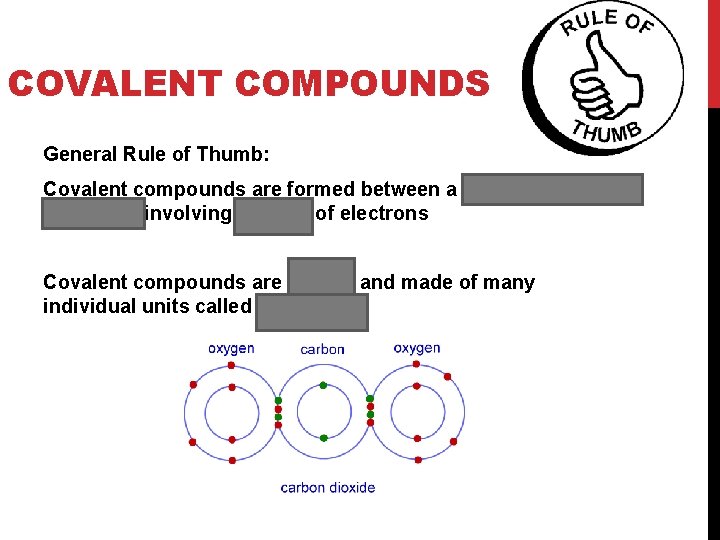
COVALENT COMPOUNDS General Rule of Thumb: Covalent compounds are formed between a non- metal and a non-metal involving sharing of electrons Covalent compounds are neutral and made of many individual units called molecules

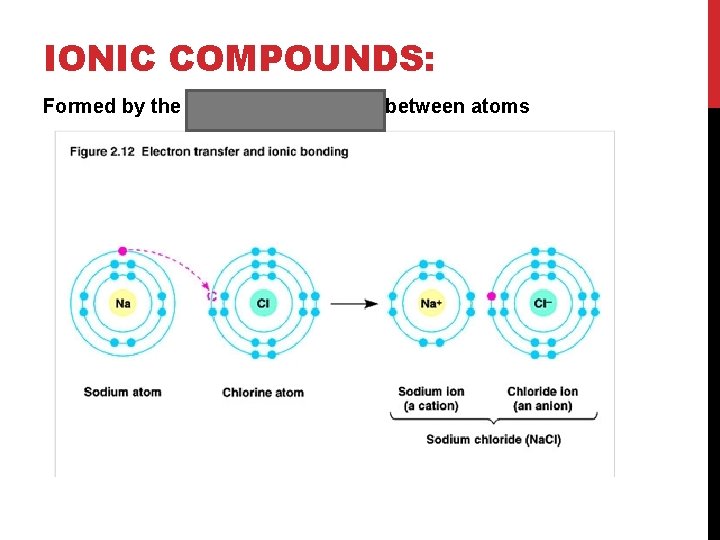
IONIC COMPOUNDS: Formed by the transfer of electrons between atoms

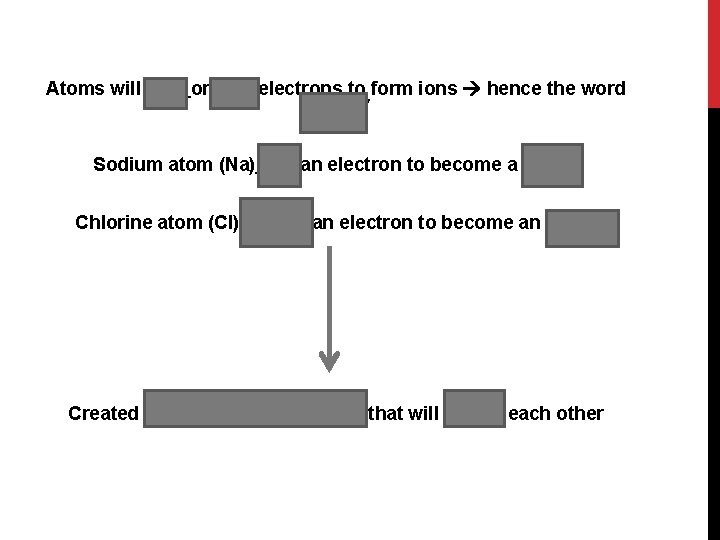
Atoms will gain or lose electrons to form ions hence the word “ionic” Sodium atom (Na) lost an electron to become a cation Chlorine atom (Cl) gained an electron to become an anion Created oppositely charged ions that will attract each other

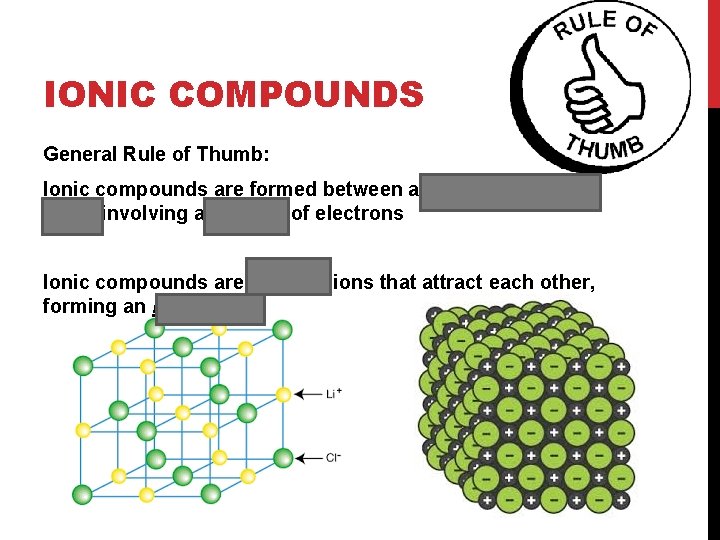
IONIC COMPOUNDS General Rule of Thumb: Ionic compounds are formed between a metal and a nonmetal involving a transfer of electrons Ionic compounds are charged ions that attract each other, forming an ionic lattice


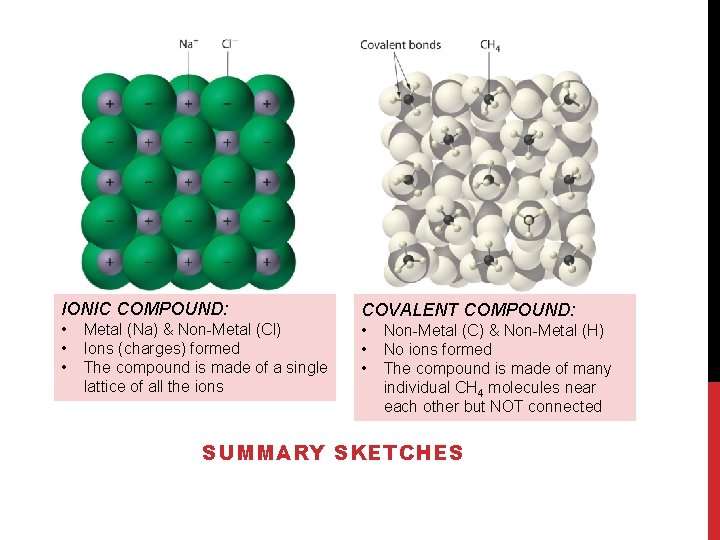
IONIC COMPOUND: • • • Metal (Na) & Non-Metal (Cl) Ions (charges) formed The compound is made of a single lattice of all the ions COVALENT COMPOUND: • • • Non-Metal (C) & Non-Metal (H) No ions formed The compound is made of many individual CH 4 molecules near each other but NOT connected SUMMARY SKETCHES