Compounds Video Clip Why it Matters NOTES 2




- Slides: 20

Compounds Video Clip: Why it Matters

NOTES 2: VALENCE ELECTRONS, LEWIS DOT DIAGRAMS, AND OXIDATION NUMBERS

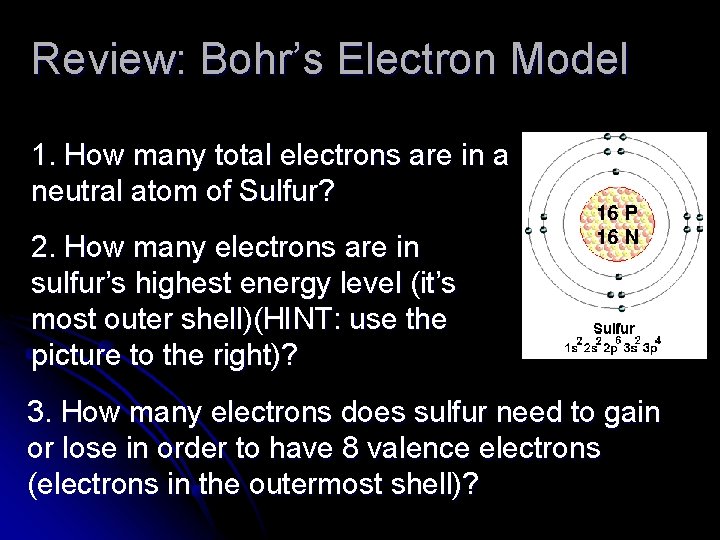
Review: Bohr’s Electron Model 1. How many total electrons are in a neutral atom of Sulfur? 2. How many electrons are in sulfur’s highest energy level (it’s most outer shell)(HINT: use the picture to the right)? 3. How many electrons does sulfur need to gain or lose in order to have 8 valence electrons (electrons in the outermost shell)?

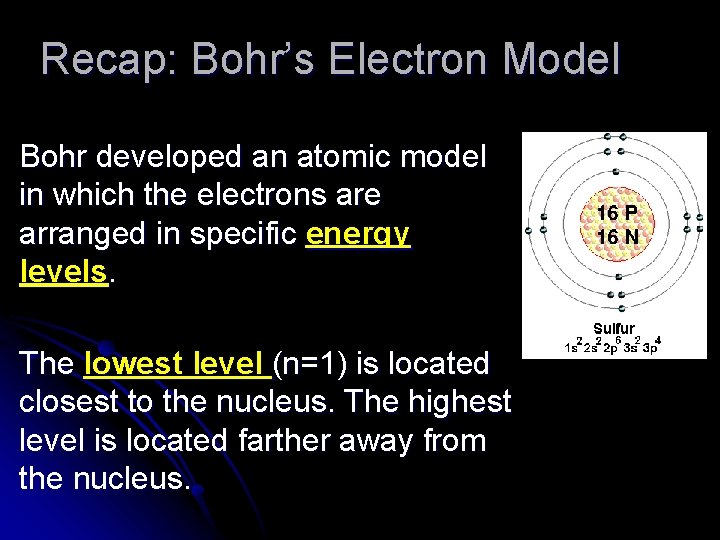
Recap: Bohr’s Electron Model Bohr developed an atomic model in which the electrons are arranged in specific energy levels. The lowest level (n=1) is located closest to the nucleus. The highest level is located farther away from the nucleus.

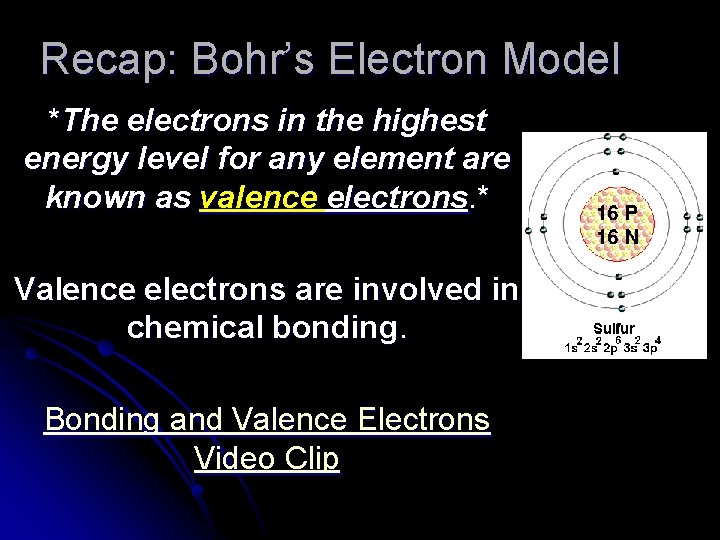
Recap: Bohr’s Electron Model *The electrons in the highest energy level for any element are known as valence electrons. * Valence electrons are involved in chemical bonding. Bonding and Valence Electrons Video Clip

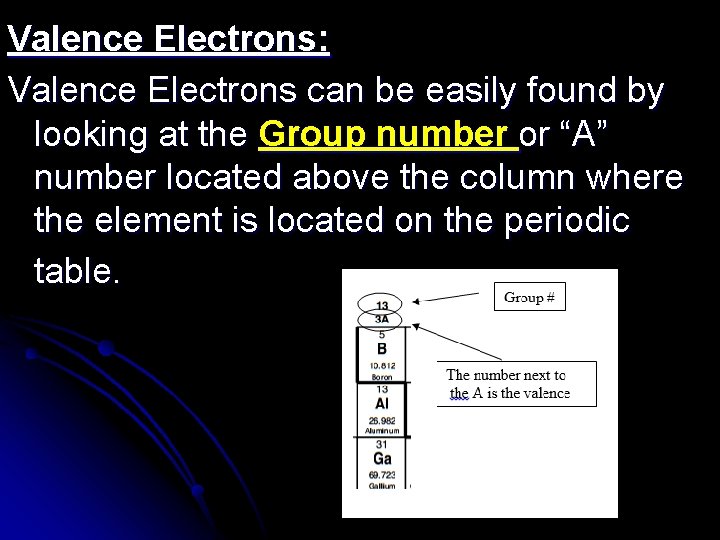
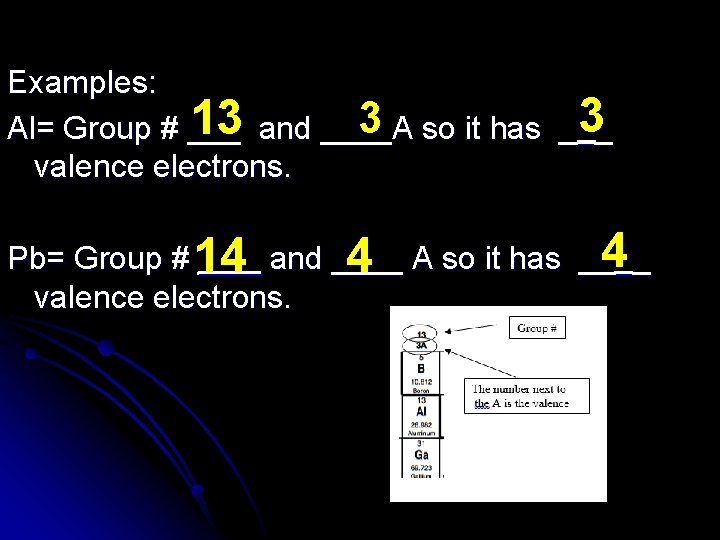
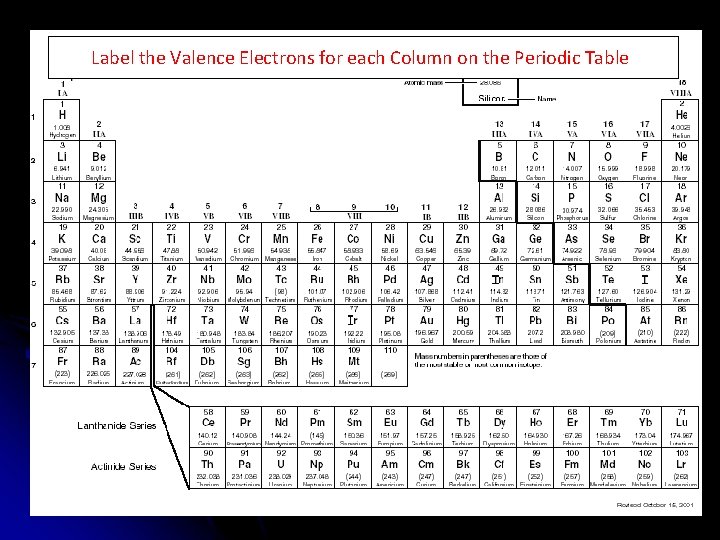
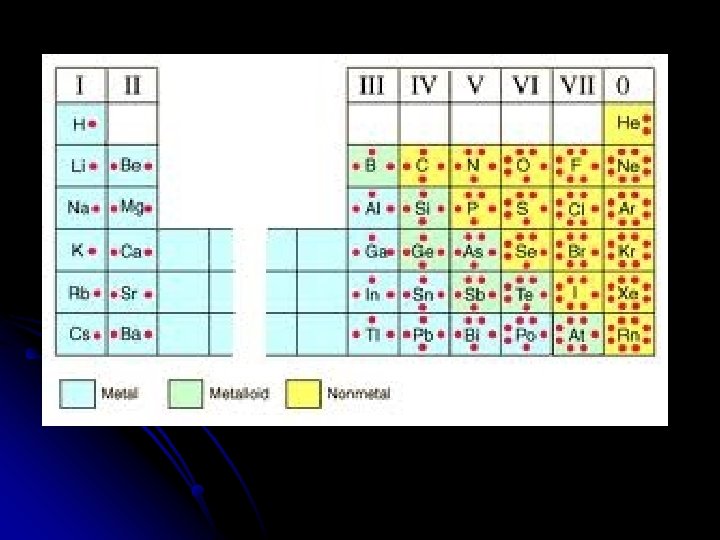
Valence Electrons: Valence Electrons can be easily found by looking at the Group number or “A” number located above the column where the element is located on the periodic table.

Examples: 3 so it has __3_ 13 and ____A Al= Group # ___ valence electrons. Pb= Group # 14 ___ and ____ 4 A so it has __4__ valence electrons.

Valence Electrons the Quick and Easy Way Determine the number of valence electrons. l. Phosphorus l. Argon l. Lead l. Barium 5 valence electrons 8 valence electrons 4 valence electrons 2 valence electrons

Exceptions • * This ONLY works for Group A Elements (the Representative Elements) • Helium two valence electrons

Label the Valence Electrons for each Column on the Periodic Table

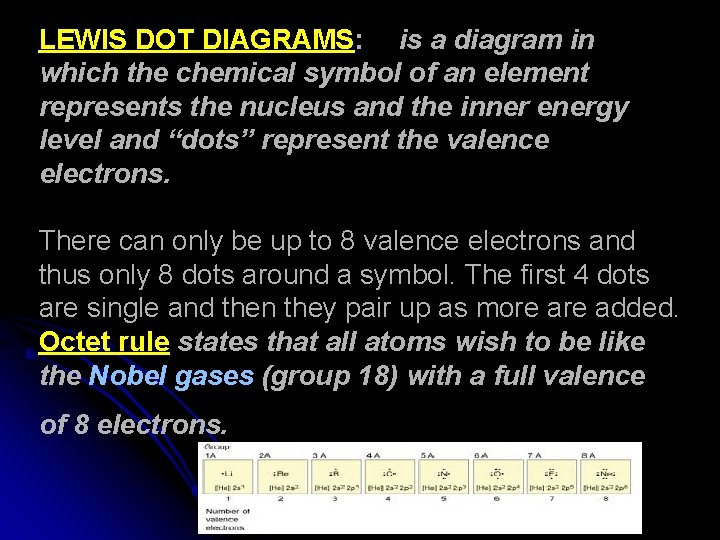
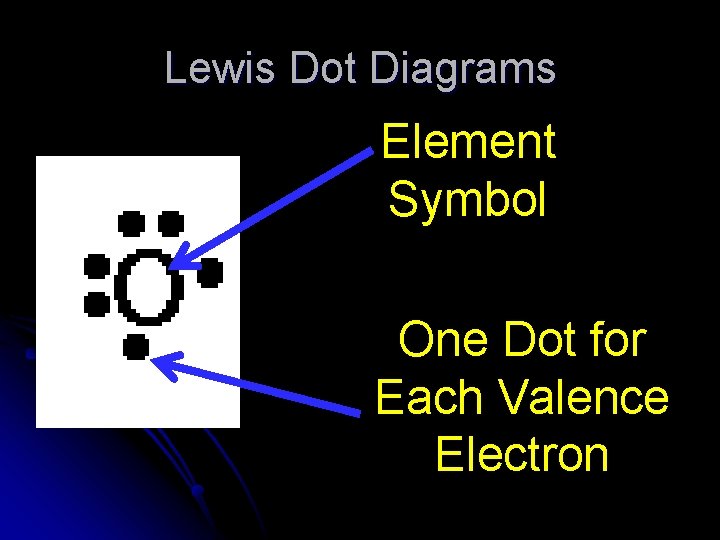
LEWIS DOT DIAGRAMS: is a diagram in which the chemical symbol of an element represents the nucleus and the inner energy level and “dots” represent the valence electrons. There can only be up to 8 valence electrons and thus only 8 dots around a symbol. The first 4 dots are single and then they pair up as more added. Octet rule states that all atoms wish to be like the Nobel gases (group 18) with a full valence of 8 electrons.


Lewis Dot Diagrams Element Symbol One Dot for Each Valence Electron

How Are the Dots Arranged? 8 4 1 5 X 7 3 2 6

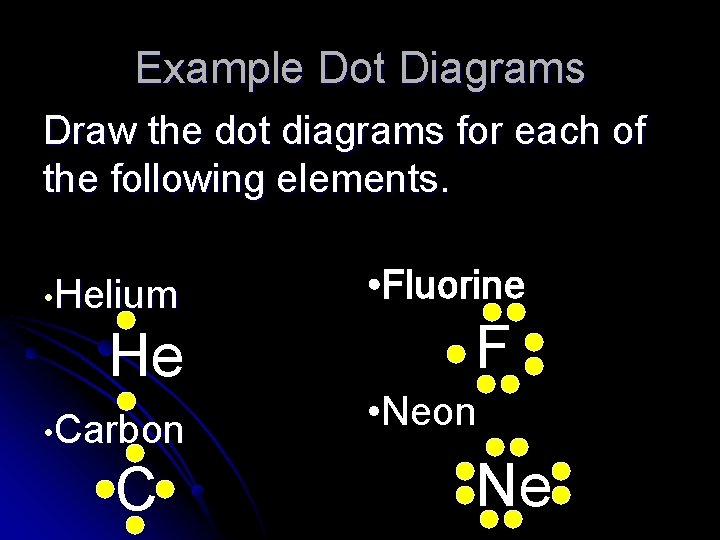
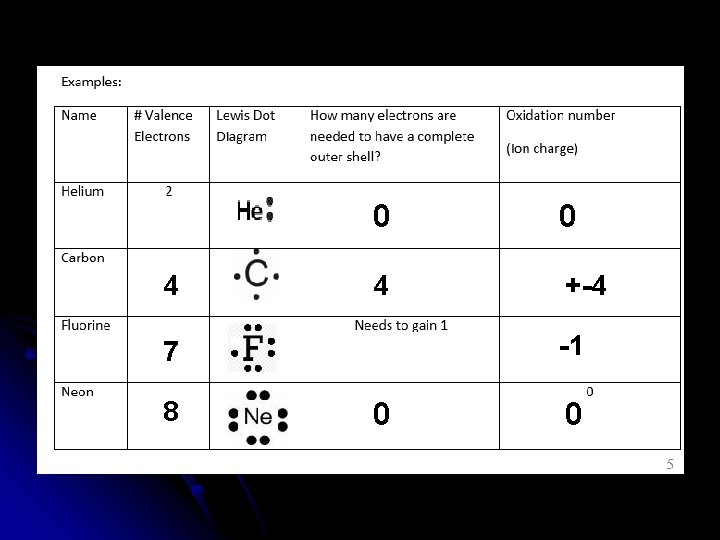
Example Dot Diagrams Draw the dot diagrams for each of the following elements. • Helium He • Carbon C • Fluorine F • Neon Ne


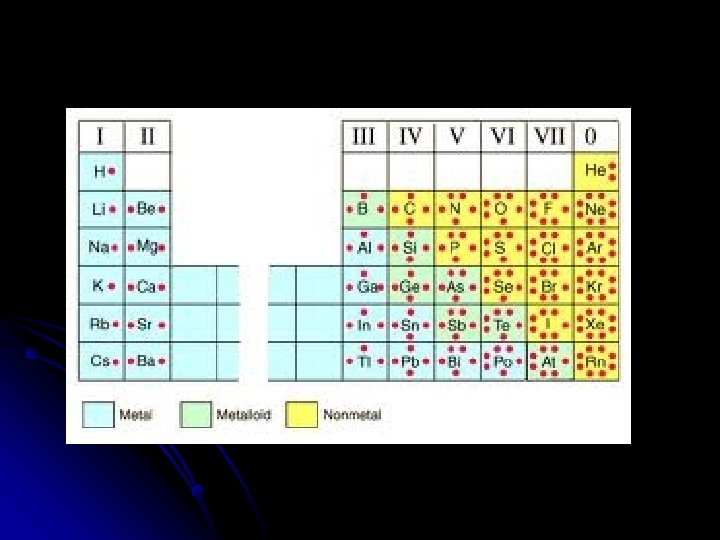
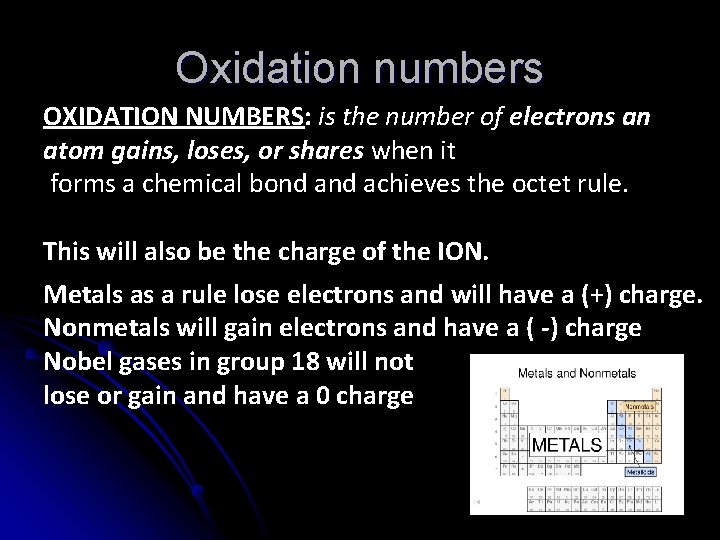
Oxidation numbers OXIDATION NUMBERS: is the number of electrons an atom gains, loses, or shares when it forms a chemical bond achieves the octet rule. This will also be the charge of the ION. Metals as a rule lose electrons and will have a (+) charge. Nonmetals will gain electrons and have a ( -) charge Nobel gases in group 18 will not lose or gain and have a 0 charge

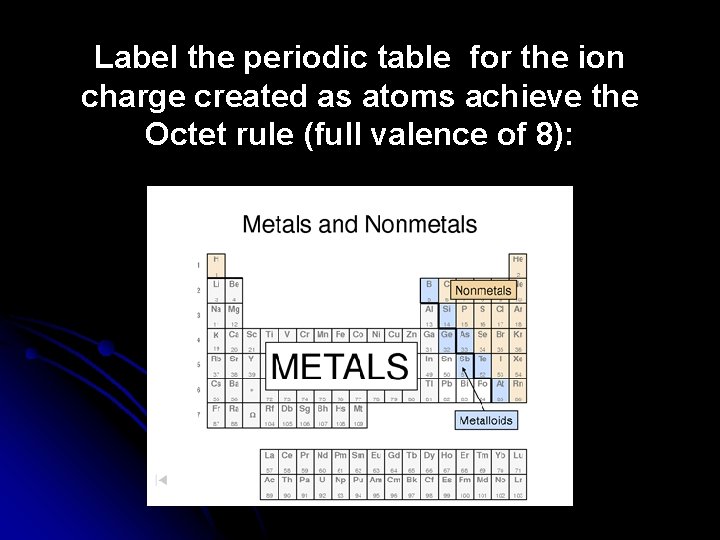
Label the periodic table for the ion charge created as atoms achieve the Octet rule (full valence of 8):

4 0 0 4 +-4 -1 7 8 0 0

Complete homework page 6