Compounds KS 3 Periodic Table Knowledge Organiser Everything
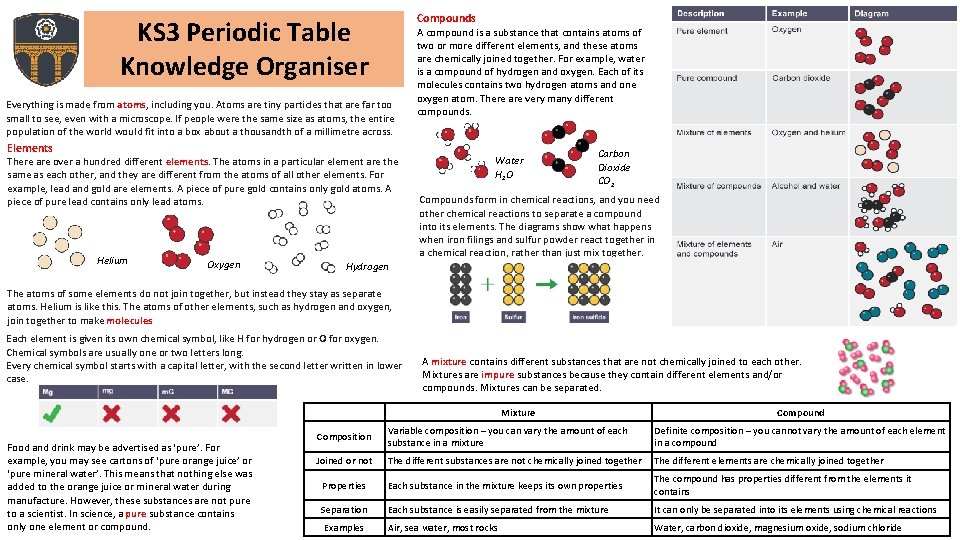
Compounds KS 3 Periodic Table Knowledge Organiser Everything is made from atoms, including you. Atoms are tiny particles that are far too small to see, even with a microscope. If people were the same size as atoms, the entire population of the world would fit into a box about a thousandth of a millimetre across. Elements There are over a hundred different elements. The atoms in a particular element are the same as each other, and they are different from the atoms of all other elements. For example, lead and gold are elements. A piece of pure gold contains only gold atoms. A piece of pure lead contains only lead atoms. Helium Oxygen A compound is a substance that contains atoms of two or more different elements, and these atoms are chemically joined together. For example, water is a compound of hydrogen and oxygen. Each of its molecules contains two hydrogen atoms and one oxygen atom. There are very many different compounds. Water H 2 O Carbon Dioxide CO 2 Compounds form in chemical reactions, and you need other chemical reactions to separate a compound into its elements. The diagrams show what happens when iron filings and sulfur powder react together in a chemical reaction, rather than just mix together. Hydrogen The atoms of some elements do not join together, but instead they stay as separate atoms. Helium is like this. The atoms of other elements, such as hydrogen and oxygen, join together to make molecules Each element is given its own chemical symbol, like H for hydrogen or O for oxygen. Chemical symbols are usually one or two letters long. Every chemical symbol starts with a capital letter, with the second letter written in lower case. A mixture contains different substances that are not chemically joined to each other. Mixtures are impure substances because they contain different elements and/or compounds. Mixtures can be separated. Mixture Food and drink may be advertised as ‘pure’. For example, you may see cartons of ‘pure orange juice’ or ‘pure mineral water’. This means that nothing else was added to the orange juice or mineral water during manufacture. However, these substances are not pure to a scientist. In science, a pure substance contains only one element or compound. Compound Composition Variable composition – you can vary the amount of each substance in a mixture Definite composition – you cannot vary the amount of each element in a compound Joined or not The different substances are not chemically joined together The different elements are chemically joined together Properties Each substance in the mixture keeps its own properties The compound has properties different from the elements it contains Separation Each substance is easily separated from the mixture It can only be separated into its elements using chemical reactions Examples Air, sea water, most rocks Water, carbon dioxide, magnesium oxide, sodium chloride
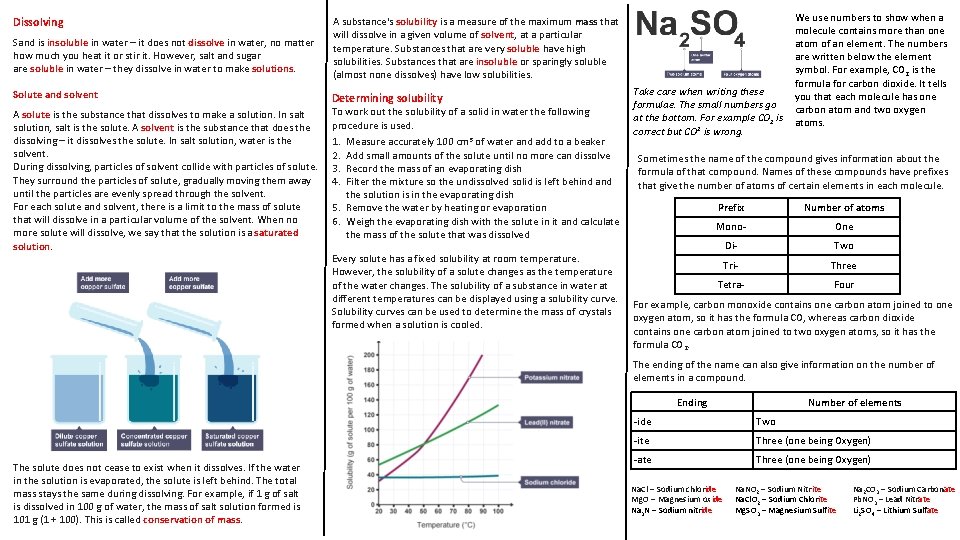
Dissolving Sand is insoluble in water – it does not dissolve in water, no matter how much you heat it or stir it. However, salt and sugar are soluble in water – they dissolve in water to make solutions. Solute and solvent A solute is the substance that dissolves to make a solution. In salt solution, salt is the solute. A solvent is the substance that does the dissolving – it dissolves the solute. In salt solution, water is the solvent. During dissolving, particles of solvent collide with particles of solute. They surround the particles of solute, gradually moving them away until the particles are evenly spread through the solvent. For each solute and solvent, there is a limit to the mass of solute that will dissolve in a particular volume of the solvent. When no more solute will dissolve, we say that the solution is a saturated solution. A substance's solubility is a measure of the maximum mass that will dissolve in a given volume of solvent, at a particular temperature. Substances that are very soluble have high solubilities. Substances that are insoluble or sparingly soluble (almost none dissolves) have low solubilities. Determining solubility To work out the solubility of a solid in water the following procedure is used. 1. 2. 3. 4. Measure accurately 100 cm 3 of water and add to a beaker Add small amounts of the solute until no more can dissolve Record the mass of an evaporating dish Filter the mixture so the undissolved solid is left behind and the solution is in the evaporating dish 5. Remove the water by heating or evaporation 6. Weigh the evaporating dish with the solute in it and calculate the mass of the solute that was dissolved Every solute has a fixed solubility at room temperature. However, the solubility of a solute changes as the temperature of the water changes. The solubility of a substance in water at different temperatures can be displayed using a solubility curve. Solubility curves can be used to determine the mass of crystals formed when a solution is cooled. Take care when writing these formulae. The small numbers go at the bottom. For example CO 2 is correct but CO 2 is wrong. We use numbers to show when a molecule contains more than one atom of an element. The numbers are written below the element symbol. For example, CO 2 is the formula for carbon dioxide. It tells you that each molecule has one carbon atom and two oxygen atoms. Sometimes the name of the compound gives information about the formula of that compound. Names of these compounds have prefixes that give the number of atoms of certain elements in each molecule. Prefix Number of atoms Mono- One Di- Two Tri- Three Tetra- Four For example, carbon monoxide contains one carbon atom joined to one oxygen atom, so it has the formula CO, whereas carbon dioxide contains one carbon atom joined to two oxygen atoms, so it has the formula CO 2. The ending of the name can also give information on the number of elements in a compound. Ending The solute does not cease to exist when it dissolves. If the water in the solution is evaporated, the solute is left behind. The total mass stays the same during dissolving. For example, if 1 g of salt is dissolved in 100 g of water, the mass of salt solution formed is 101 g (1 + 100). This is called conservation of mass. Number of elements -ide Two -ite Three (one being Oxygen) -ate Three (one being Oxygen) Na. Cl – Sodium chloride Mg. O – Magnesium oxide Na 3 N – Sodium nitride Na. NO 2 – Sodium Nitrite Na. Cl. O 2 – Sodium Chlorite Mg. SO 3 – Magnesium Sulfite Na 2 CO 3 – Sodium Carbonate Pb. NO 3 – Lead Nitrate Li 2 SO 4 – Lithium Sulfate
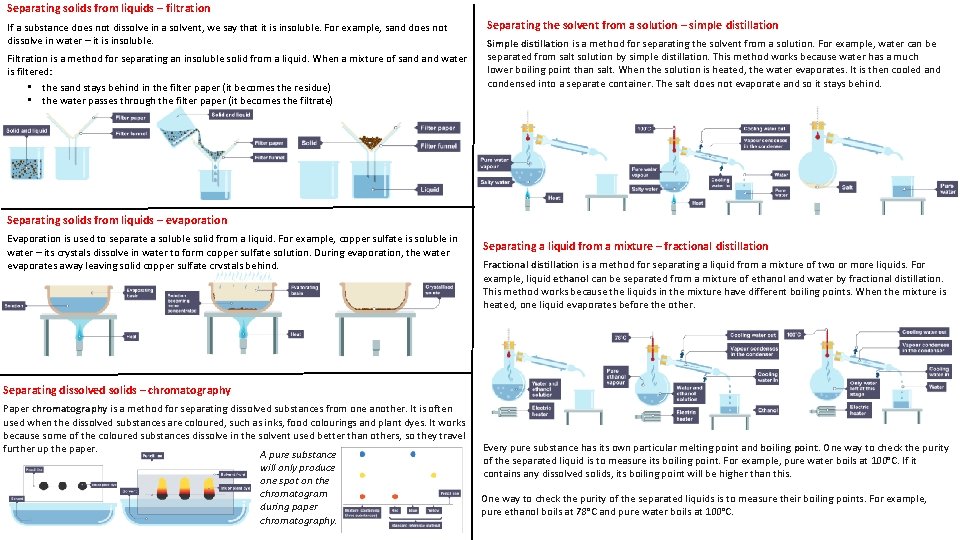
Separating solids from liquids – filtration If a substance does not dissolve in a solvent, we say that it is insoluble. For example, sand does not dissolve in water – it is insoluble. Filtration is a method for separating an insoluble solid from a liquid. When a mixture of sand water is filtered: • the sand stays behind in the filter paper (it becomes the residue) • the water passes through the filter paper (it becomes the filtrate) Separating the solvent from a solution – simple distillation Simple distillation is a method for separating the solvent from a solution. For example, water can be separated from salt solution by simple distillation. This method works because water has a much lower boiling point than salt. When the solution is heated, the water evaporates. It is then cooled and condensed into a separate container. The salt does not evaporate and so it stays behind. Separating solids from liquids – evaporation Evaporation is used to separate a soluble solid from a liquid. For example, copper sulfate is soluble in water – its crystals dissolve in water to form copper sulfate solution. During evaporation, the water evaporates away leaving solid copper sulfate crystals behind. Separating a liquid from a mixture – fractional distillation Fractional distillation is a method for separating a liquid from a mixture of two or more liquids. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. This method works because the liquids in the mixture have different boiling points. When the mixture is heated, one liquid evaporates before the other. Separating dissolved solids – chromatography Paper chromatography is a method for separating dissolved substances from one another. It is often used when the dissolved substances are coloured, such as inks, food colourings and plant dyes. It works because some of the coloured substances dissolve in the solvent used better than others, so they travel further up the paper. A pure substance will only produce one spot on the chromatogram during paper chromatography. Every pure substance has its own particular melting point and boiling point. One way to check the purity of the separated liquid is to measure its boiling point. For example, pure water boils at 100°C. If it contains any dissolved solids, its boiling point will be higher than this. One way to check the purity of the separated liquids is to measure their boiling points. For example, pure ethanol boils at 78°C and pure water boils at 100°C.

The periodic table The modern periodic table is based closely on the ideas he used: • the elements are arranged in order of increasing atomic number • the horizontal rows are called periods • the vertical columns are called groups • elements in the same group are similar to each other History of the periodic table Newlands’ octaves An English scientist called John Newlands put forward his Law of Octaves in 1864. He arranged all the elements known at the time into a table in order of relative atomic mass. When he did this, he found a pattern among the early elements. The pattern showed that each element was similar to the element eight places ahead of it. For example, starting at Li (lithium), Be (beryllium) is the second element, B (boron) is the third and Na (sodium) is the eighth element. He then put the similar elements into vertical columns, known as groups. Mendeleev's periodic table The main groups are numbered from 1 to 7 going from left to right, and the last group on the right is group 0. The section in the middle of the table is called the Transition Metals. You may also see all the groups numbered (including the transition metals), this time from 1 to 18. If you know what one of the elements in a group is like, you can make predictions about the other elements in a group. For example, all the elements in group 1 are reactivemetals, and all the elements in group 0 are unreactive non-metals. The zig-zag line in this diagram separates the metals, on the left, from non-metals, on the right. Hydrogen is a non-metal but it is often put in the middle. Notice that most elements are metals, rather than non-metals. Each element has its own chemical symbol, made from letters. Remember that you will only find elements in the periodic table and never compounds. In 1869, just five years after John Newlands put forward his Law of Octaves, a Russian chemist called Dmitri Mendeleev published a periodic table. Mendeleev also arranged the elements known at the time in order of relative atomic mass, but he did some other things that made his table much more successful. He realised that the physical and chemical properties of elements were related to their atomic mass in a repeating or 'periodic' way, and arranged them so that groups of elements with similar properties fell into vertical columns in his table. Making predictions using the periodic table Groups in the periodic table contain elements with similar chemical properties. But there are usually trends in properties that allow us to make predictions. For example, in group 1: Melting point Density Reactivity Lithium Decreases down the group Increases down the group Sodium Decreases down the group Increases down the group Decreases Potassium down the group Increases down the group Decreases down the group Increases down the group Rubidium • Releases hydrogen gas, skates around on top of the water. • Becomes smaller until it disappears • Releases heat and can spark • Leaves an alkaline hydroxide Caesium is the next element in group 1, and it can be found below rubidium. You can accurately predict that it will have the lowest melting point, the highest density and the highest reactivity of all the elements in group 1.
- Slides: 4