Compounds Ionic Compounds made by combination of metal

Compounds

Ionic Compounds • made by combination of metal and nonmetal • metal loses electrons – forms cation • nonmetal gains electrons – forms anion • consider Na and S – Na+ and S 2 - ions formed – pack together to make compound Na 2 S
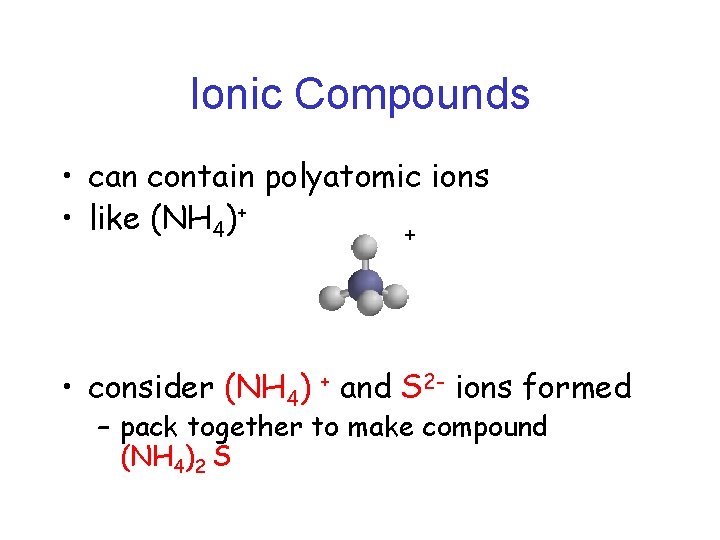
Ionic Compounds • can contain polyatomic ions • like (NH 4)+ + • consider (NH 4) + and S 2 - ions formed – pack together to make compound (NH 4)2 S
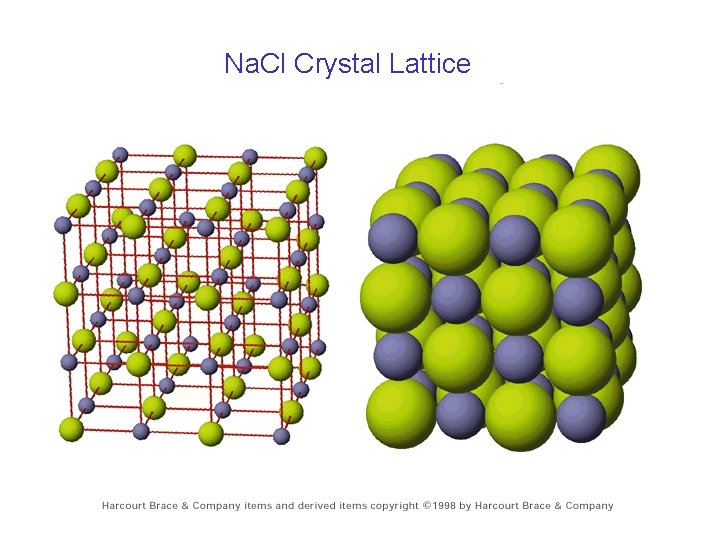
Na. Cl Crystal Lattice

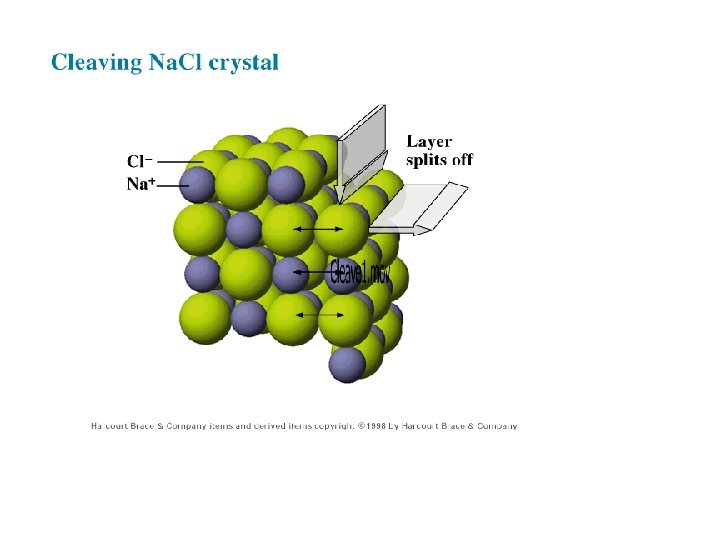
Properties of Ionic Compounds • • high melting point hard but brittle cleave with flat edges when dissolve in water, dissolves as ions • Conductors when melted or dissolved in water

Covalent (Molecular) Compounds • made by combination of nonmetals only • made by “sharing” electrons • make molecules…fundamental particle of compound
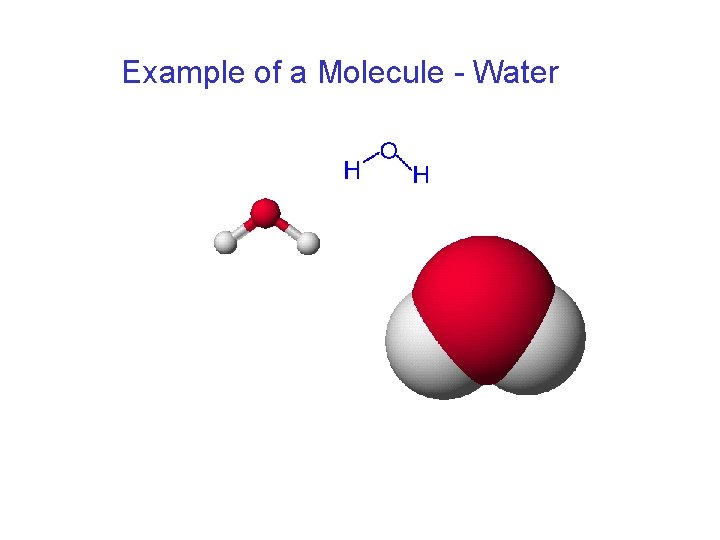
Example of a Molecule - Water
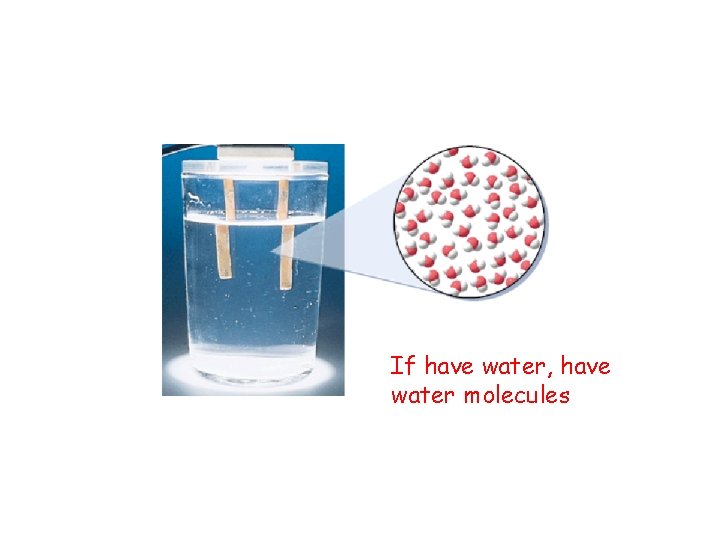
If have water, have water molecules
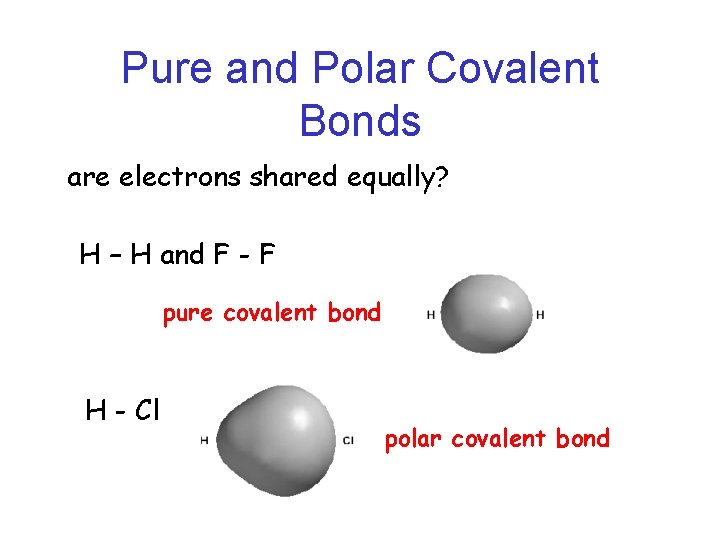
Pure and Polar Covalent Bonds are electrons shared equally? H – H and F - F pure covalent bond H - Cl polar covalent bond
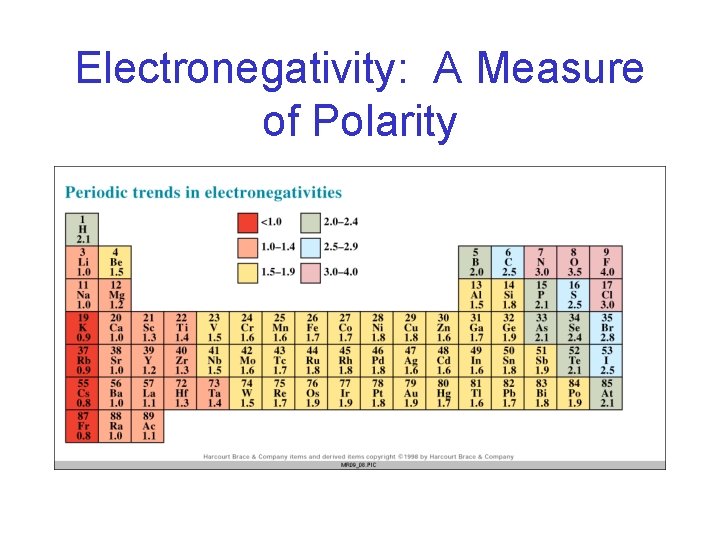
Electronegativity: A Measure of Polarity
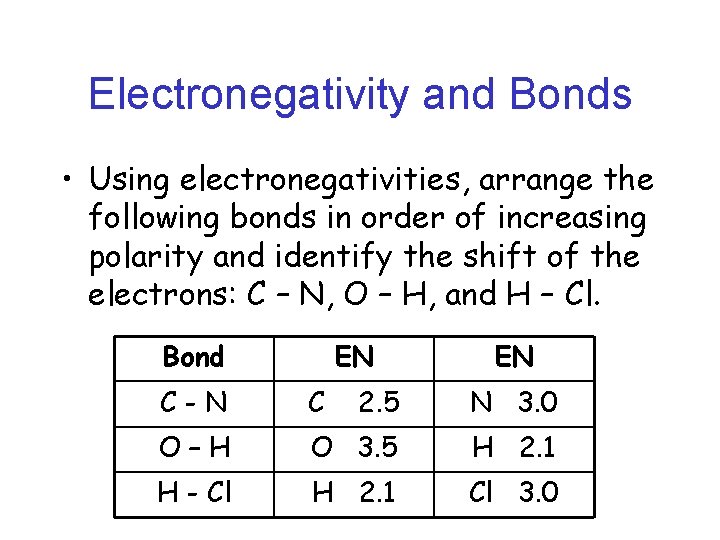
Electronegativity and Bonds • Using electronegativities, arrange the following bonds in order of increasing polarity and identify the shift of the electrons: C – N, O – H, and H – Cl. Bond EN EN C-N C 2. 5 N 3. 0 O–H O 3. 5 H 2. 1 H - Cl H 2. 1 Cl 3. 0

Electronegativity and Bonds • Without using the numbers in the electronegativity scale, determine which would be more ionic Li. F or BF 3?
- Slides: 14