Compounds Formulas Compounds There about 100 million pure

Compounds & Formulas

Compounds • There about 100 million pure substances that have been identified • Out of these pure substances, only 118 of them are elements, the rest are compounds • Compounds are made up of more than one element that is held together by a chemical bond • Every compound is made up of a chemical formula
Chemical Formulas • A chemical formula tells us: • A) the type of atoms present • B) the number of atoms present • C) the type of compound

Reading Chemical Formulas • E. g. table salt: Sodium Chloride • Chemical formula Na. Cl • Count the atoms present – 1 Na atom – 1 Cl atom
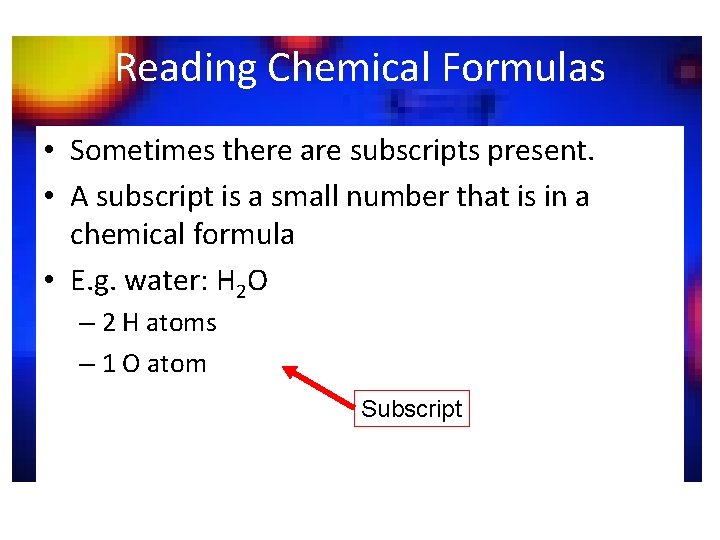
Reading Chemical Formulas • Sometimes there are subscripts present. • A subscript is a small number that is in a chemical formula • E. g. water: H 2 O – 2 H atoms – 1 O atom Subscript
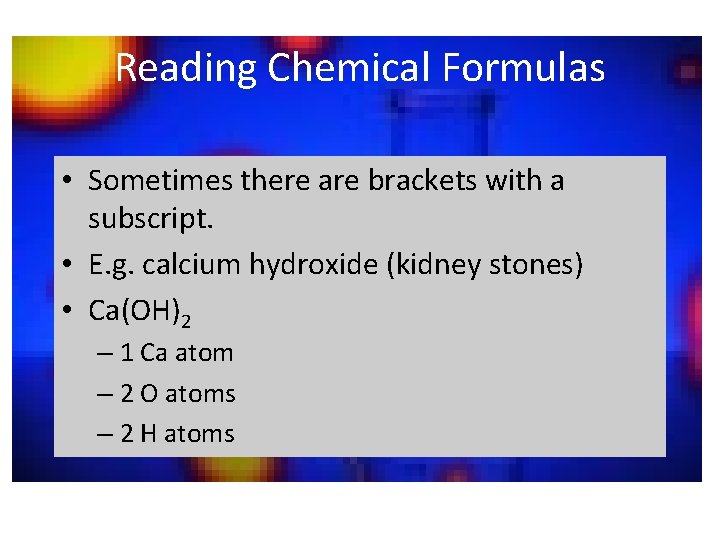
Reading Chemical Formulas • Sometimes there are brackets with a subscript. • E. g. calcium hydroxide (kidney stones) • Ca(OH)2 – 1 Ca atom – 2 O atoms – 2 H atoms
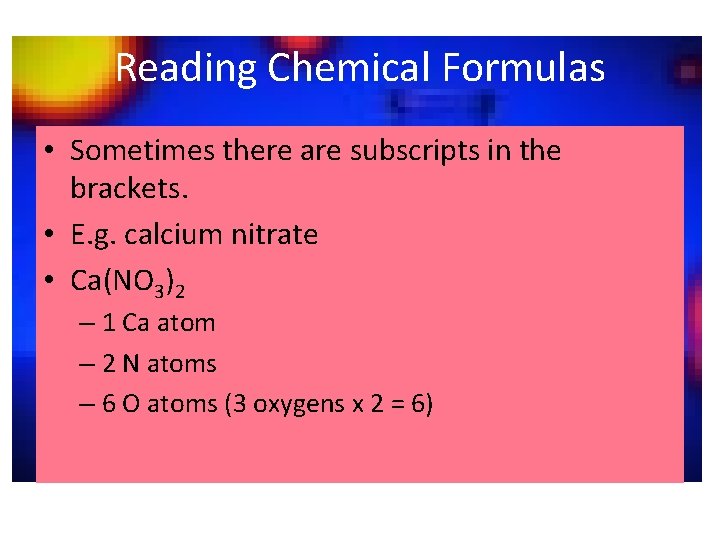
Reading Chemical Formulas • Sometimes there are subscripts in the brackets. • E. g. calcium nitrate • Ca(NO 3)2 – 1 Ca atom – 2 N atoms – 6 O atoms (3 oxygens x 2 = 6)

Assignment • • How to Count Atoms Review Counting Atoms Worksheet Subscripts and Coefficients Chemical Bonding definitions
- Slides: 8