Compounds Compound l a substance made up of
Compounds

Compound: l a substance made up of atoms of different elements joined together (involves a chemical change)

Example: l *There is no atom of water. l *Two atoms of hydrogen join with one atom of oxygen. (chemical bond) l *The smallest unit of water is called a molecule.
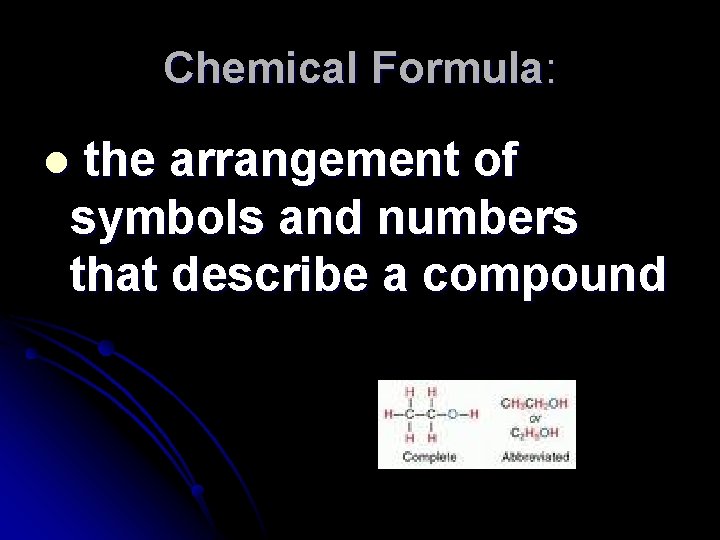
Chemical Formula: the arrangement of symbols and numbers that describe a compound l

Example: l H 2 O l H = Hydrogen l How many atoms of hydrogen? 2 atoms of hydrogen

O = Oxygen • If there is no number behind the element symbol, there is just one atom of that element. • Therefore, there is only 1 atom of oxygen in H 20.
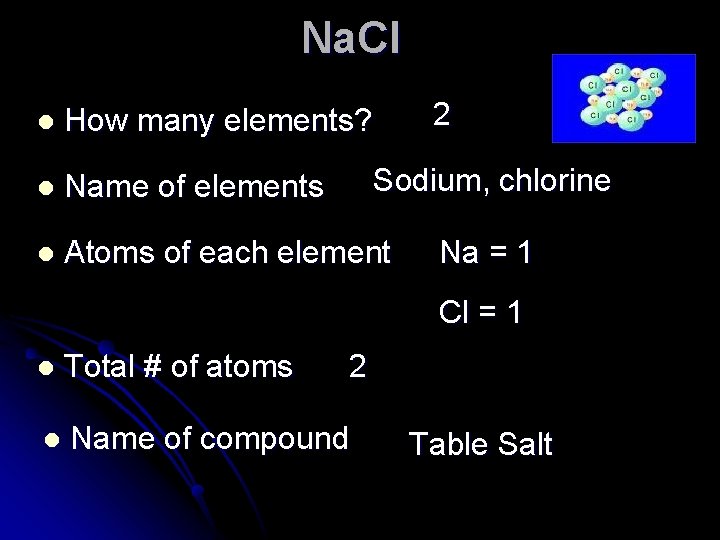
Na. Cl 2 l How many elements? l Name of elements l Atoms of each element Sodium, chlorine Na = 1 Cl = 1 l Total # of atoms 2 l Name of compound Table Salt
Your Turn

Other Chemical Formulas

nitrogen dioxide l one atom of nitrogen l two atoms of oxygen NO 2

aluminum oxide l two atoms of aluminum l three atoms of oxygen Al 2 O 3
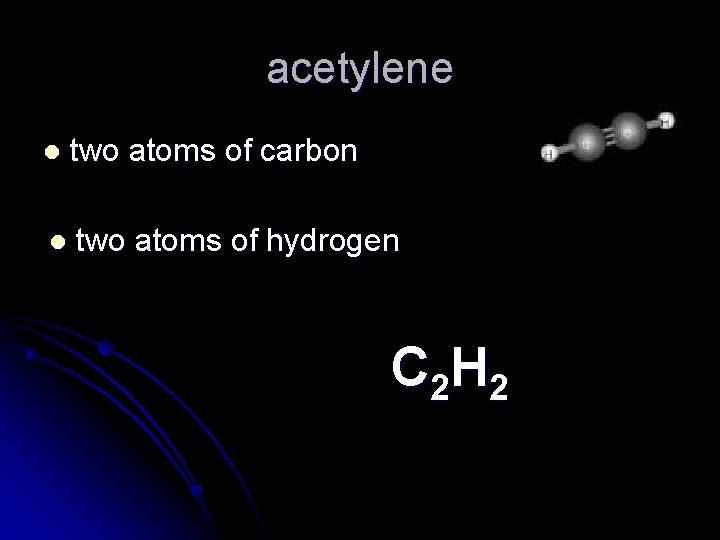
acetylene l two atoms of carbon l two atoms of hydrogen C 2 H 2
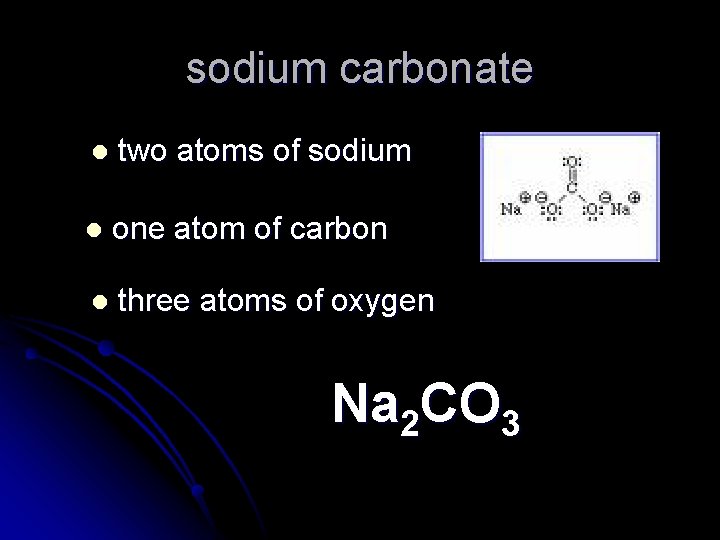
sodium carbonate l two atoms of sodium l one atom of carbon l three atoms of oxygen Na 2 CO 3
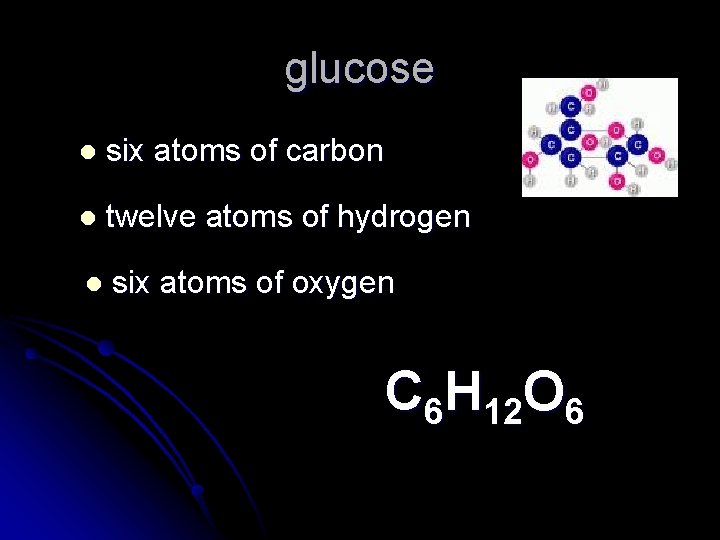
glucose l six atoms of carbon l twelve atoms of hydrogen l six atoms of oxygen C 6 H 12 O 6
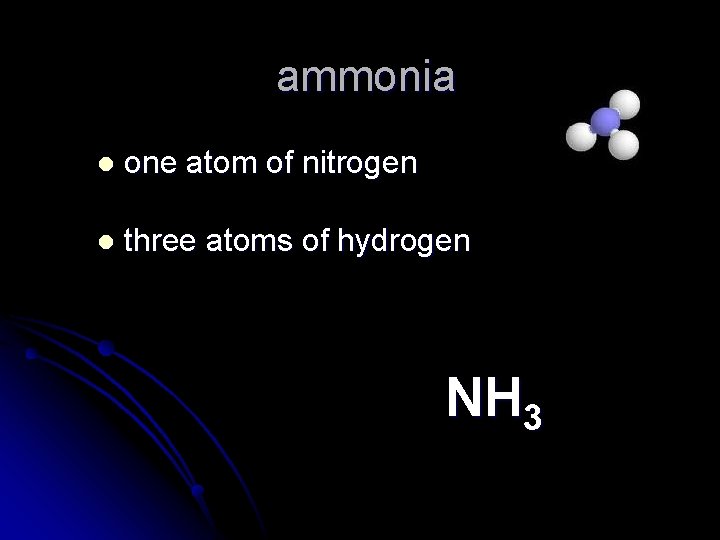
ammonia l one atom of nitrogen l three atoms of hydrogen NH 3
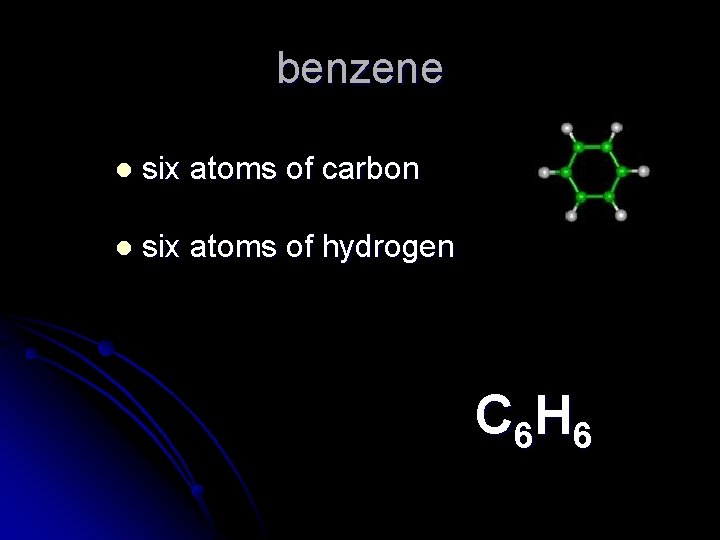
benzene l six atoms of carbon l six atoms of hydrogen C 6 H 6

Recipes for Compound Molecules

Write the following as a formula: # 1. hydrogen peroxide l two atoms of hydrogen, two atoms of oxygen H 2 O 2

Here are some formulas. You write out the recipe.

# 1. aluminum phosphate Al. PO 4 l one atom of aluminum l one atom of phosphorus l four atoms of oxygen

Do you know how to read formulas? Read through the following formulas for molecules of different compounds. Then tell how many different elements and atoms are in each molecule.

# 4. Fe 2 O 3 l # of elements 2 l # of atoms 5 l common name rust

Click for Video Clip on Compounds
- Slides: 23