Compounds Chemical Bonds Chemical Formulas When chemical bonds

Compounds & Chemical Bonds
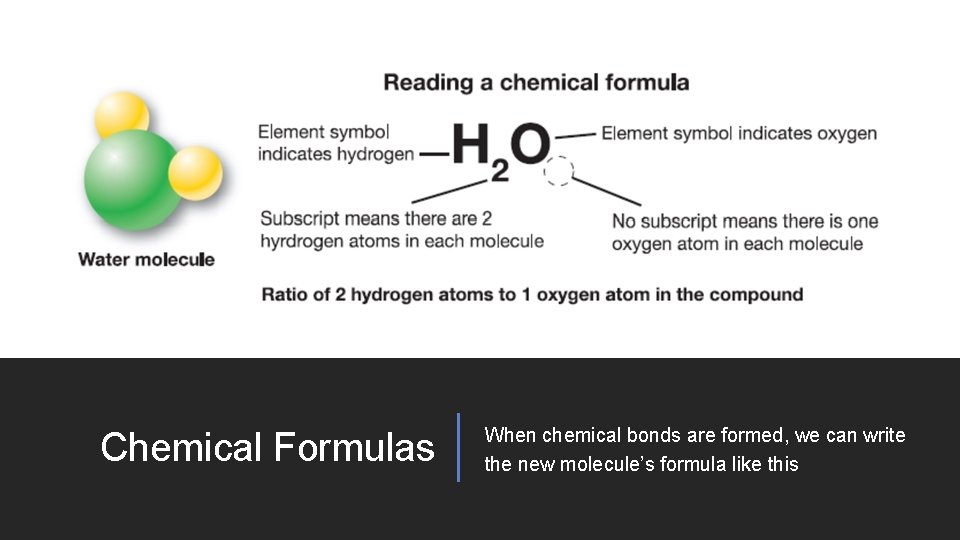
Chemical Formulas When chemical bonds are formed, we can write the new molecule’s formula like this
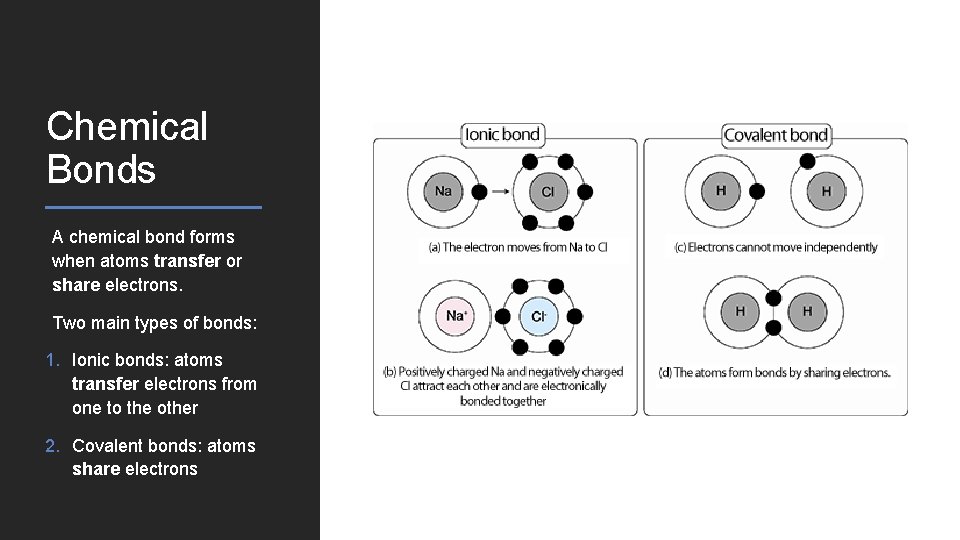
Chemical Bonds A chemical bond forms when atoms transfer or share electrons. Two main types of bonds: 1. Ionic bonds: atoms transfer electrons from one to the other 2. Covalent bonds: atoms share electrons
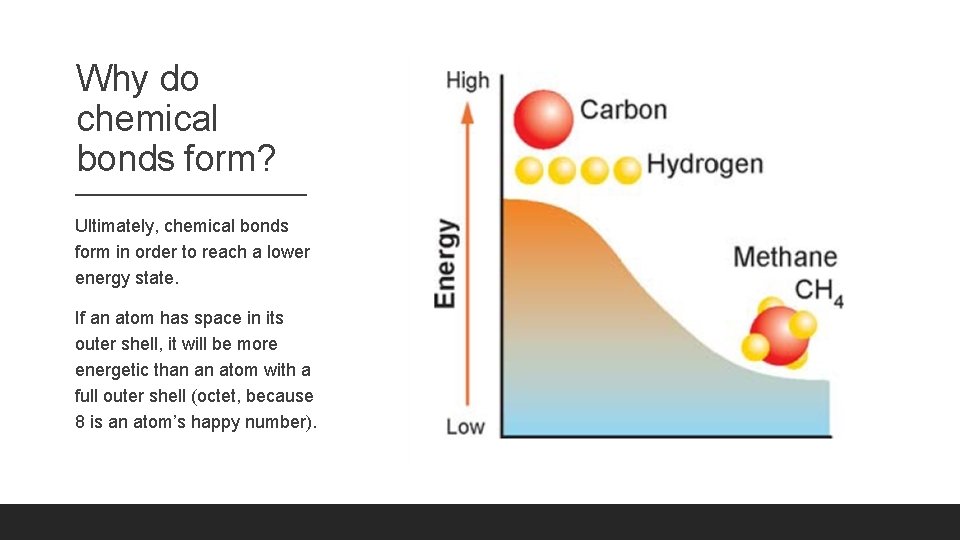
Why do chemical bonds form? Ultimately, chemical bonds form in order to reach a lower energy state. If an atom has space in its outer shell, it will be more energetic than an atom with a full outer shell (octet, because 8 is an atom’s happy number).
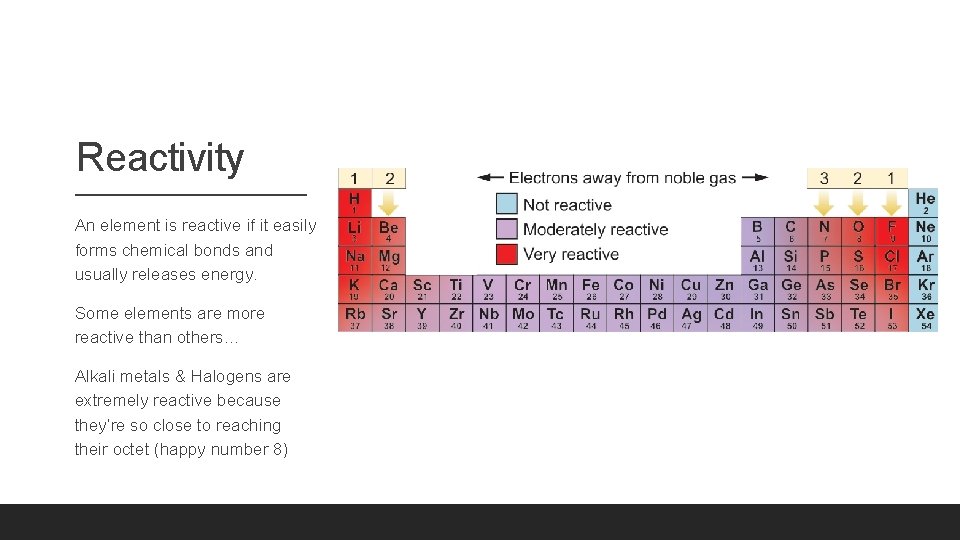
Reactivity An element is reactive if it easily forms chemical bonds and usually releases energy. Some elements are more reactive than others… Alkali metals & Halogens are extremely reactive because they’re so close to reaching their octet (happy number 8)
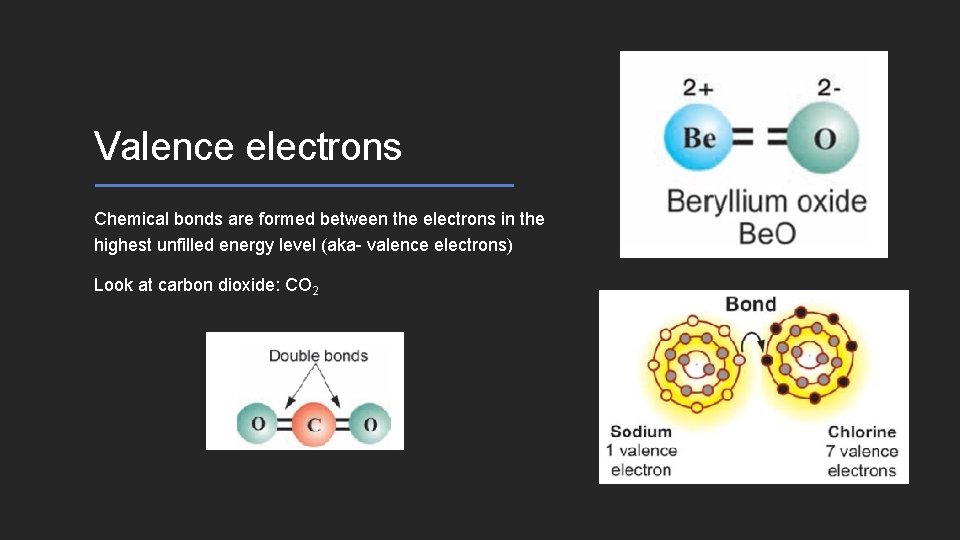
Valence electrons Chemical bonds are formed between the electrons in the highest unfilled energy level (aka- valence electrons) Look at carbon dioxide: CO 2
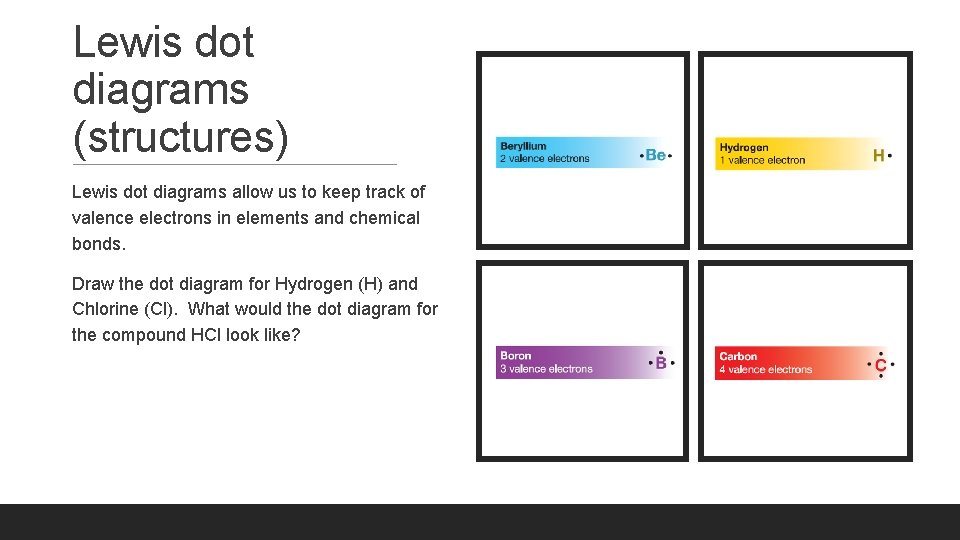
Lewis dot diagrams (structures) Lewis dot diagrams allow us to keep track of valence electrons in elements and chemical bonds. Draw the dot diagram for Hydrogen (H) and Chlorine (Cl). What would the dot diagram for the compound HCl look like?
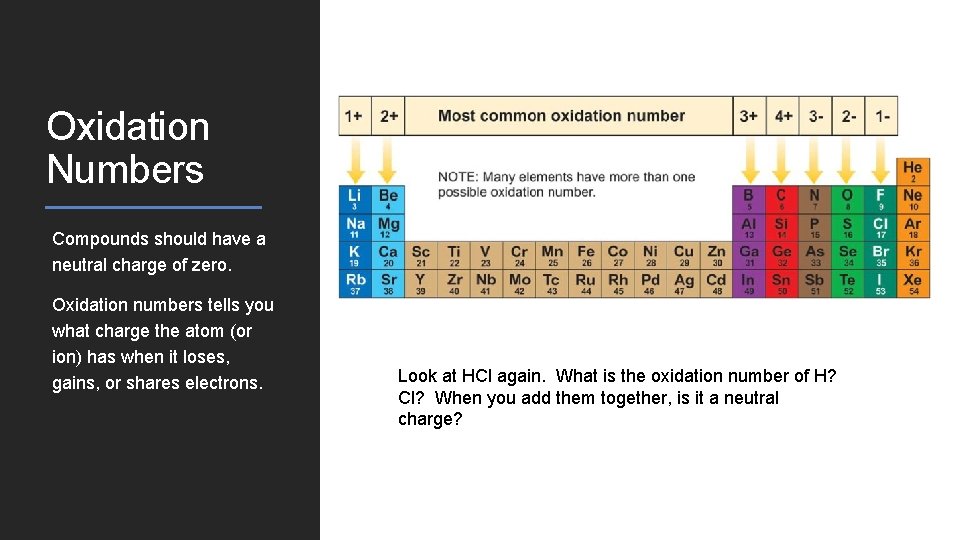
Oxidation Numbers Compounds should have a neutral charge of zero. Oxidation numbers tells you what charge the atom (or ion) has when it loses, gains, or shares electrons. Look at HCl again. What is the oxidation number of H? Cl? When you add them together, is it a neutral charge?
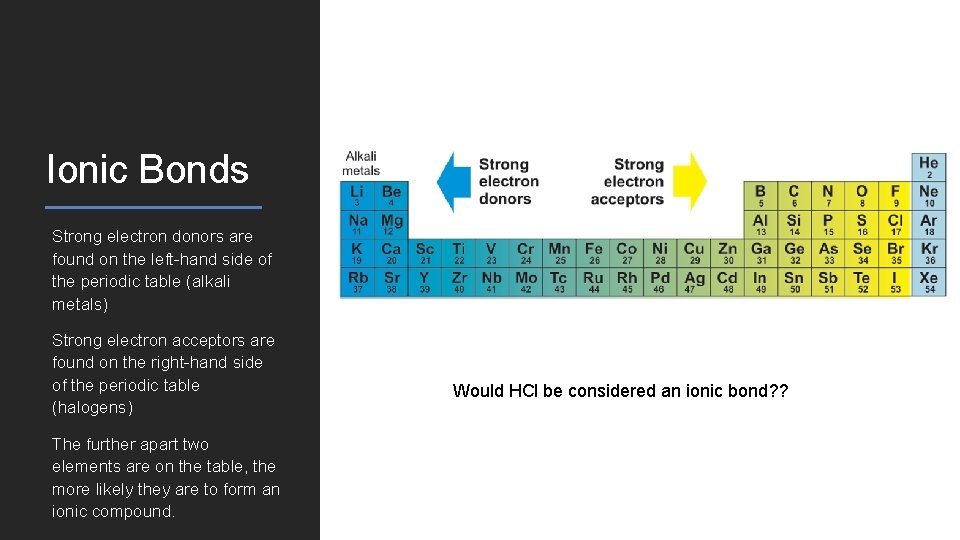
Ionic Bonds Strong electron donors are found on the left-hand side of the periodic table (alkali metals) Strong electron acceptors are found on the right-hand side of the periodic table (halogens) The further apart two elements are on the table, the more likely they are to form an ionic compound. Would HCl be considered an ionic bond? ?
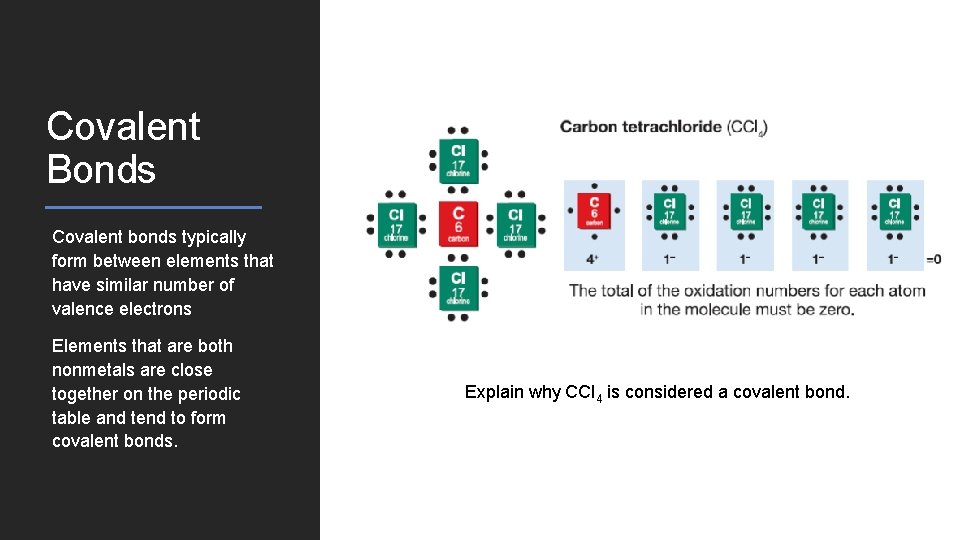
Covalent Bonds Covalent bonds typically form between elements that have similar number of valence electrons Elements that are both nonmetals are close together on the periodic table and tend to form covalent bonds. Explain why CCl 4 is considered a covalent bond.

How to write a chemical formula. 1. Find the oxidation number of each element in the compound. ◦ Ex. Carbon is 4+ and Oxygen is 2 -, so we would have C 4+ and O 2 - 2. Determine the ratios of each element and write chemical formula. ◦ In order for us to have a neutral charge between Carbon and Oxygen, we would need ONE Carbon (4+) and TWO Oxygen (2 -) C 4 - + O 2+ = 0 3. Write the chemical formula. ◦ CO 2 TRY IT with Beryllium and Oxygen.
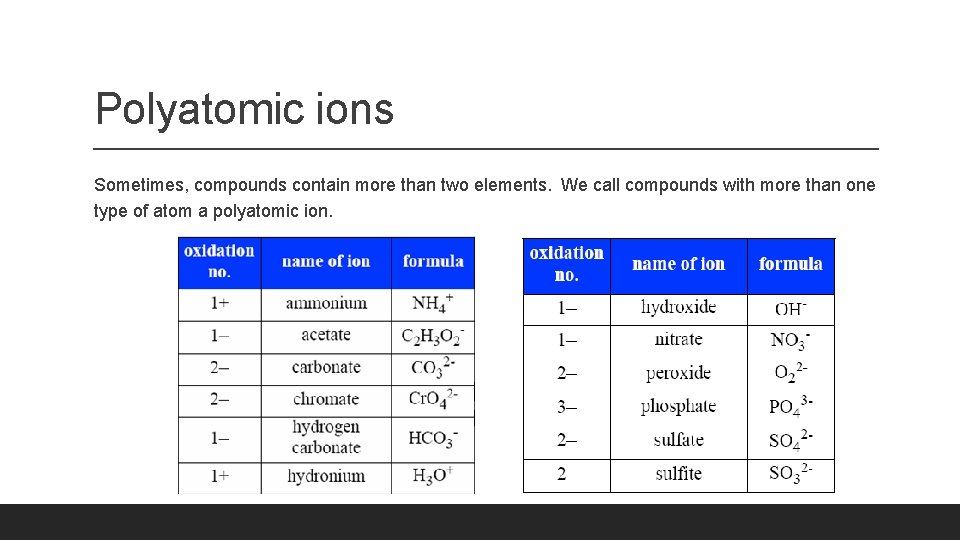
Polyatomic ions Sometimes, compounds contain more than two elements. We call compounds with more than one type of atom a polyatomic ion.
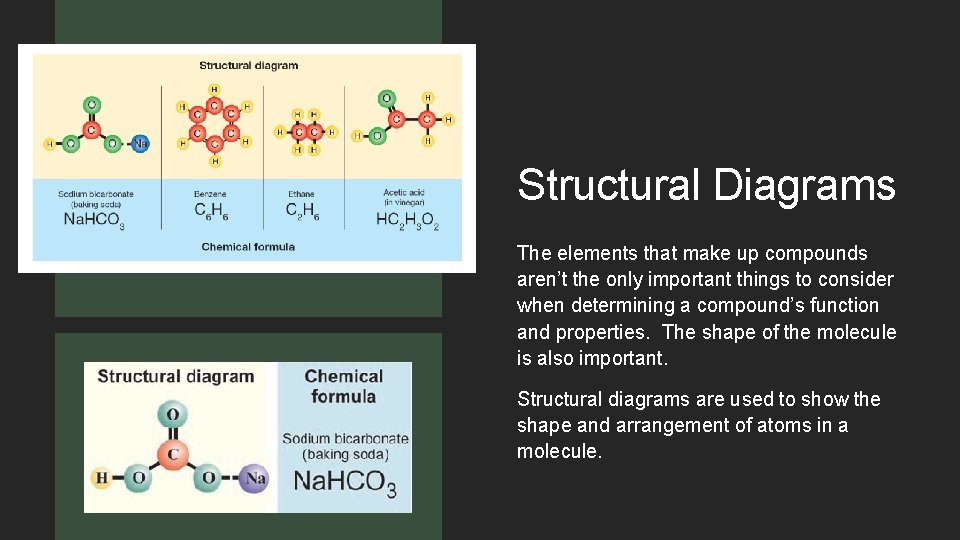
Structural Diagrams The elements that make up compounds aren’t the only important things to consider when determining a compound’s function and properties. The shape of the molecule is also important. Structural diagrams are used to show the shape and arrangement of atoms in a molecule.
- Slides: 13