Compounds Bonding Crystals Standards Classify matter in a

Compounds, Bonding & Crystals

Standards Ø Classify matter in a variety of ways (element, compound…) Ø Explain how electrons determine the properties of substances by: • Interactions between atoms through transferring or sharing valence electrons • Ionic and covalent bonds Ø Make predictions about elements using the periodic table (e. g. , number of valence electrons, type of bond between elements) Ø Understand how the type and arrangement of atoms and their bonds determine macroscopic properties (e. g. , properties of minerals, such as hardness)

Compound • Two or more atoms that are chemically bonded together. • Remember atoms are the smallest piece of an element, which are found on the periodic table. • Minerals are compounds. • Examples: 1. Quartz: Si. O 2 this is 1 Si atom bonded to 2 oxygen atoms (the subscript denotes how many atoms of each element, no subscript means 1 atom) 2. Halite (table salt): Na. Cl, this is 1 Na atom bonded to 1 Cl atom
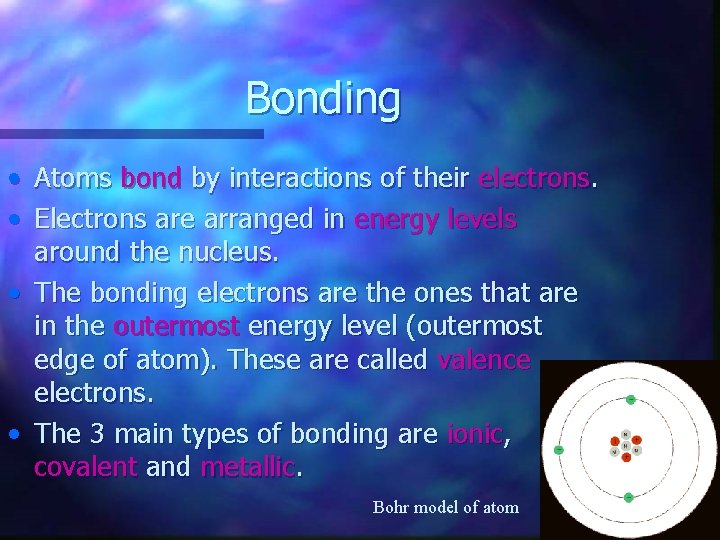
Bonding • • Atoms bond by interactions of their electrons. Electrons are arranged in energy levels around the nucleus. • The bonding electrons are the ones that are in the outermost energy level (outermost edge of atom). These are called valence electrons. • The 3 main types of bonding are ionic, covalent and metallic. Bohr model of atom

Valence Electrons • Can be determined for some of the elements by looking at the periodic table. • Vertical columns are called groups, and are numbered from 1 to 18.

Valence Electrons • The valence electrons for elements in groups 1 & 2 are equal to the group number. • Valence electrons for elements in groups 13 -18 are equal to the group number minus 10. • Valence electrons can’t be determined from periodic table for groups 3 -12.

Bonding • Atoms bond because they want to have a full outer energy level, or 8 valence electrons. • They want to be like the noble gases in group 18, which have a full outer energy level.

Ionic Bonding • Occurs by transfer of electrons from one atom to another. • Atoms gain or lose electrons. • Elements in groups 1, 2 & 13 lose 1, 2 or 3 electrons respectively. • Elements in groups 15, 16 & 17 gain 3, 2 or 1 electrons respectively. • Usually occurs between metals and nonmetals
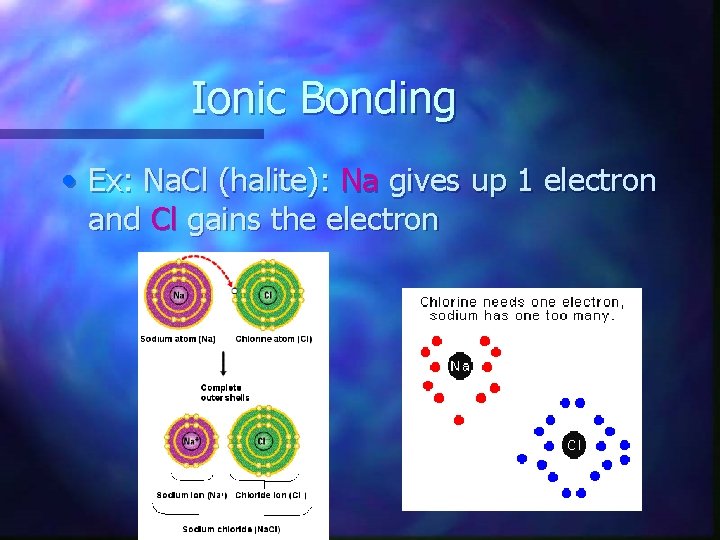
Ionic Bonding • Ex: Na. Cl (halite): Na gives up 1 electron and Cl gains the electron

Covalent Bonding • Two atoms share electrons to get a total of 8 valence electrons (with the exception of hydrogen & helium, which only have 2). • Usually a combination of nonmetals. • Electrons are shared in pairs.
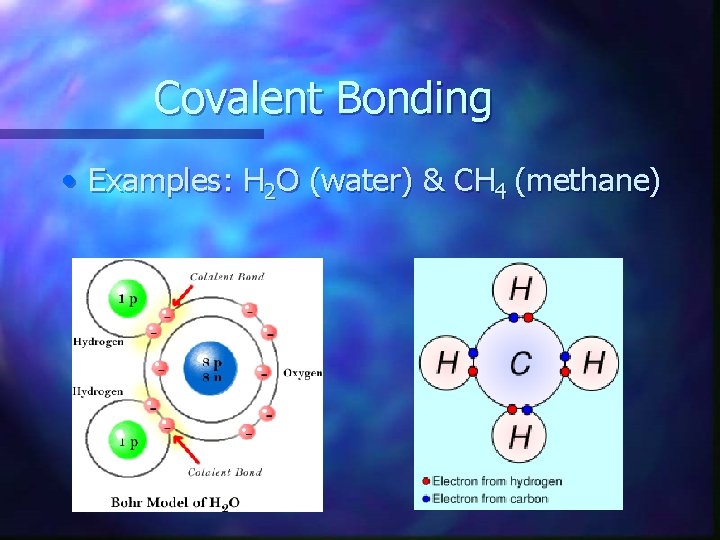
Covalent Bonding • Examples: H 2 O (water) & CH 4 (methane)

Metallic Bonding • Several metal atoms share electrons: “sea” of electrons. • Electrons are free to move throughout metal structure from atom to atom. • Occurs in metals.

Metallic Bonding • Example: Cu (copper), Au (gold), Ag (silver), Fe (iron)

Why is Bonding Important to Crystals & Minerals? • The way atoms are bonded in a crystal determines the shape of the crystal. • If a crystal grows slowly and with lots of room, it will have a crystal form that mimics the arrangement of its atoms, causing it to have good crystal faces. Ex: quartz point • If there is not enough space, or crystallization is too fast, the crystals grow over one another and form a solid mass (which we call massive). Ex: chunk of quartz

Why is Bonding Important to Crystals & Minerals? • Bonding also gives crystals and minerals their properties. • Ex: diamonds and graphite are both made of carbon atoms. Diamonds are hard because of the way the carbon atoms covalently bond into a 3 -d lattice, while graphite is soft because the carbon atoms bond in sheets that are held together by weak bonds.

Minerals, crystals and Gemstones
- Slides: 16