Compounds and Their Bonds Shapes and Polarity of
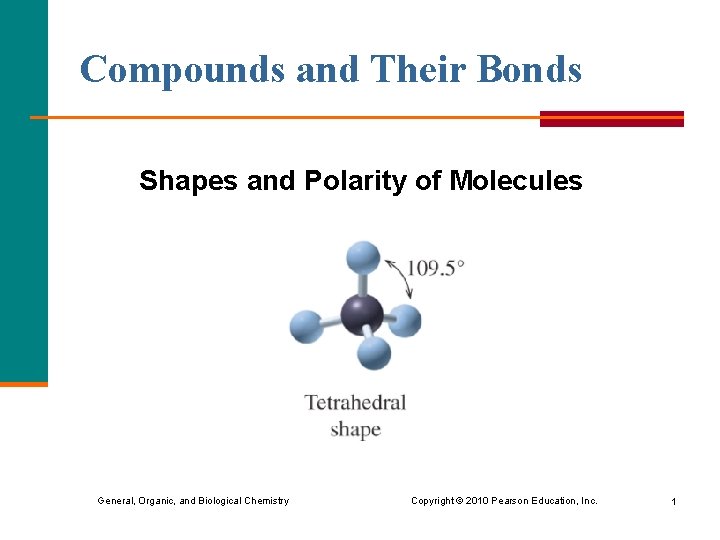
Compounds and Their Bonds Shapes and Polarity of Molecules General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 1
VSEPR In the valence-shell electron-pair repulsion theory (VSEPR), the electron groups around a central atom § are arranged as far apart from each other as possible § have the least amount of repulsion of the negatively charged electrons § have a geometry around the central atom that determines molecular shape General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 2
Shapes of Molecules The three-dimensional shape of a molecule § is the result of bonded groups and lone pairs of electrons around the central atom § is predicted using the VSEPR theory (valence-shellelectron-pair repulsion) General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 3
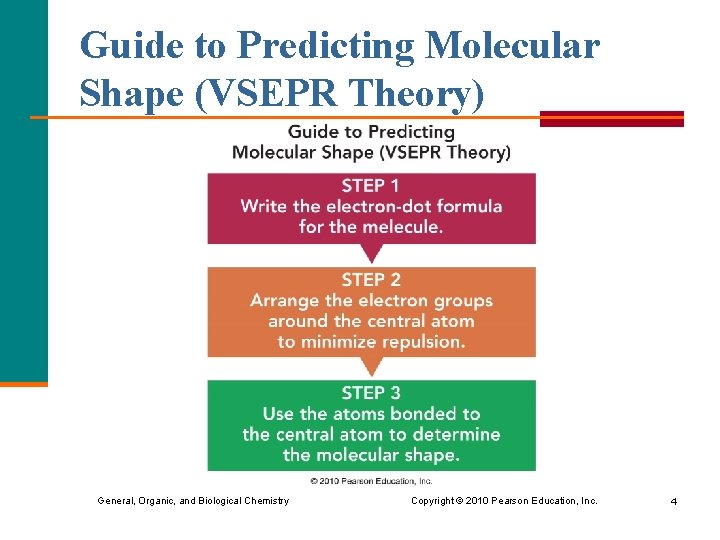
Guide to Predicting Molecular Shape (VSEPR Theory) General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 4
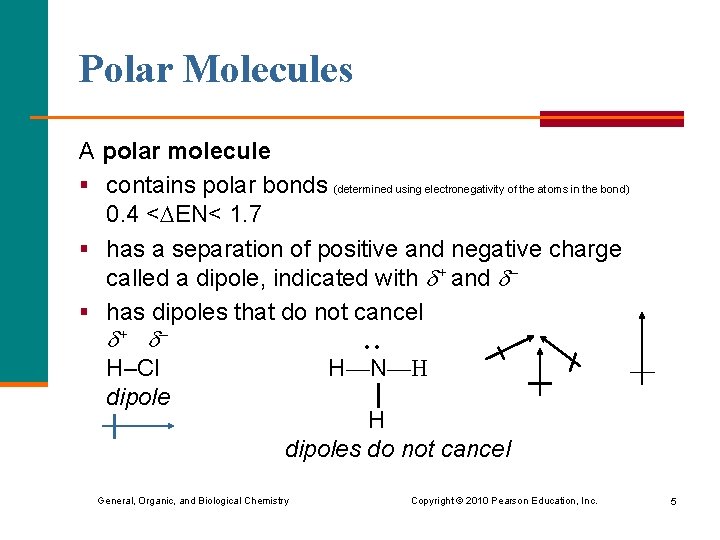
Polar Molecules A polar molecule § contains polar bonds (determined using electronegativity of the atoms in the bond) 0. 4 < EN< 1. 7 § has a separation of positive and negative charge called a dipole, indicated with + and – § has dipoles that do not cancel + – H–Cl dipole • • H—N—H dipoles do not cancel General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 5
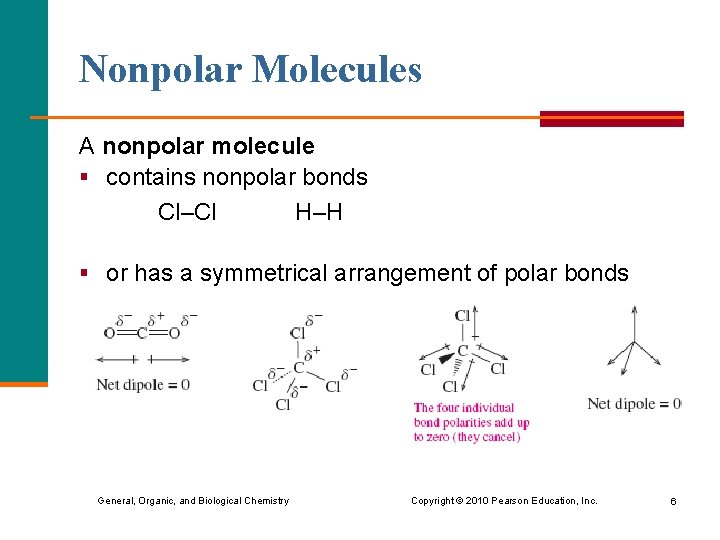
Nonpolar Molecules A nonpolar molecule § contains nonpolar bonds Cl–Cl H–H § or has a symmetrical arrangement of polar bonds General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 6
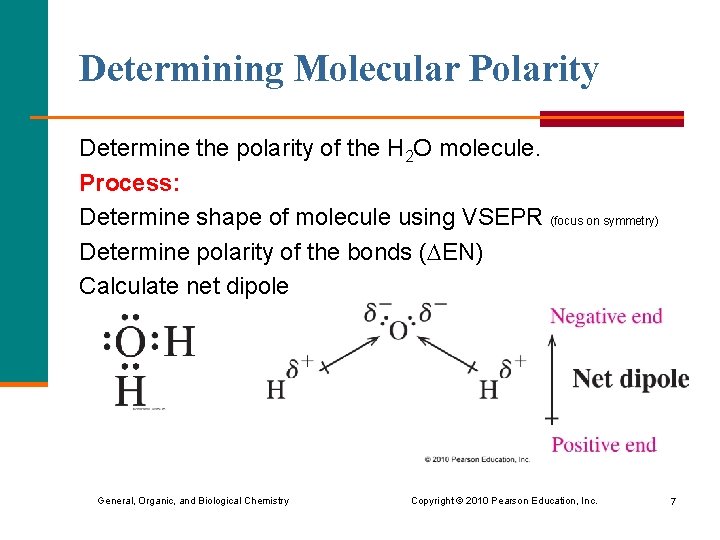
Determining Molecular Polarity Determine the polarity of the H 2 O molecule. Process: Determine shape of molecule using VSEPR (focus on symmetry) Determine polarity of the bonds ( EN) Calculate net dipole General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 7
Learning Check Determine the shape of each of the following molecules and whether they are polar or nonpolar. Explain. A. PBr 3 B. HBr C. Br 2 D. Si. Br 4 General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 8

Solution A) pyramidal; dipoles don’t cancel; polar B) linear; one polar bond (dipole); polar C) linear; nonpolar bond; nonpolar D) tetrahedral; dipoles cancel; nonpolar General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 9
Summary n A molecule will be polar if it is asymmetrical AND contains polar bonds. n A molecule will be non-polar if it is asymmetrical BUT contains non-polar bonds. n A molecule will be non-polar if it is symmetrical, regardless of bond type. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 10
What Good is This? ? n The polarity of a molecule will tell you a lot about properties such as solubility, boiling/melting points, etc. when you compare it to other similar molecules. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 11
VSEPR Theory and Polarity n Complete the worksheet from yesterday by determining if the molecules/ions are polar or non-polar. n Read section 4. 5 n Attempt some problems from the electronic problem set. (see unit page) n Complete Page 227 #1 -3 General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 12
- Slides: 12