Compounds and Their Bonds Electronegativity and Bond Polarity
Compounds and Their Bonds Electronegativity and Bond Polarity Bond energies Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 1

Electronegativity The electronegativity value • indicates the attraction of an atom for shared electrons. • increases from left to right going across a period on the periodic table. • is high for the nonmetals with fluorine as the highest. • is low for the metals. 2
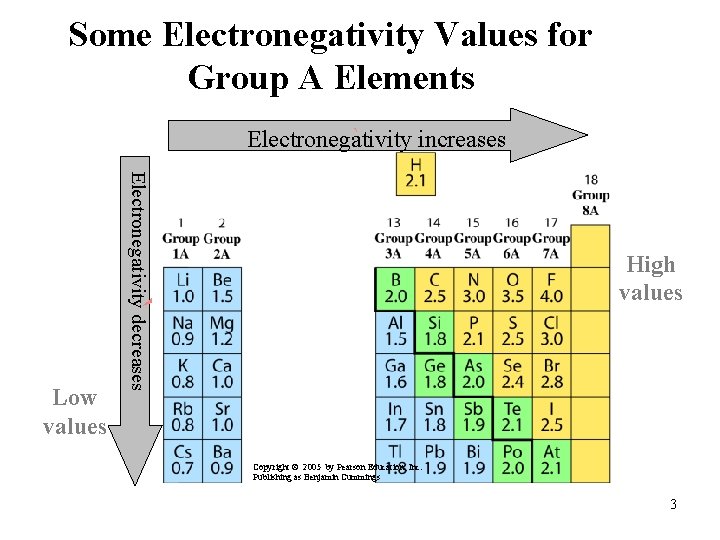
Some Electronegativity Values for Group A Elements ` Electronegativity increases Electronegativity decreases ` High values Low values Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 3
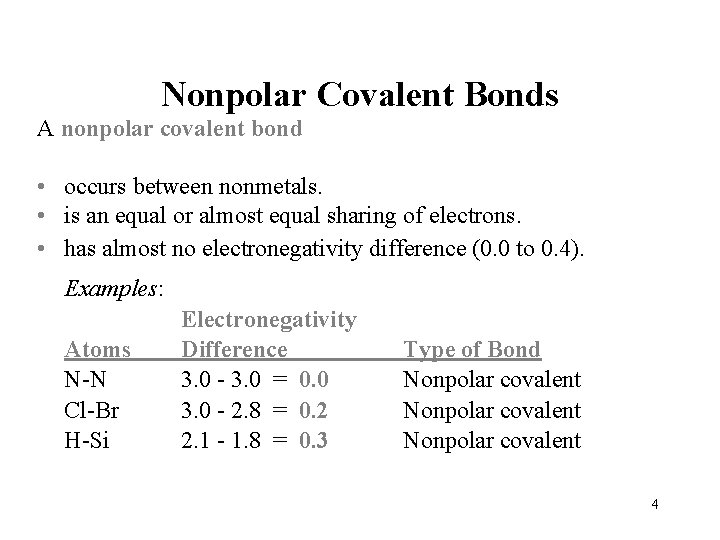
Nonpolar Covalent Bonds A nonpolar covalent bond • occurs between nonmetals. • is an equal or almost equal sharing of electrons. • has almost no electronegativity difference (0. 0 to 0. 4). Examples: Atoms N-N Cl-Br H-Si Electronegativity Difference 3. 0 - 3. 0 = 0. 0 3. 0 - 2. 8 = 0. 2 2. 1 - 1. 8 = 0. 3 Type of Bond Nonpolar covalent 4
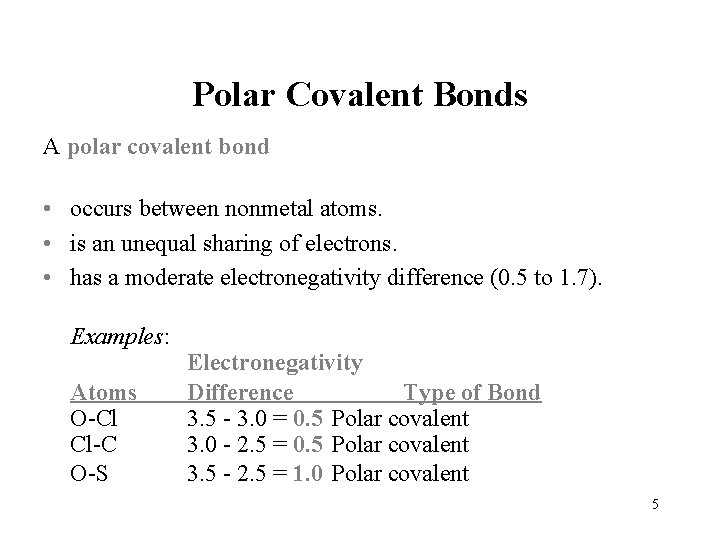
Polar Covalent Bonds A polar covalent bond • occurs between nonmetal atoms. • is an unequal sharing of electrons. • has a moderate electronegativity difference (0. 5 to 1. 7). Examples: Atoms O-Cl Cl-C O-S Electronegativity Difference Type of Bond 3. 5 - 3. 0 = 0. 5 Polar covalent 3. 0 - 2. 5 = 0. 5 Polar covalent 3. 5 - 2. 5 = 1. 0 Polar covalent 5
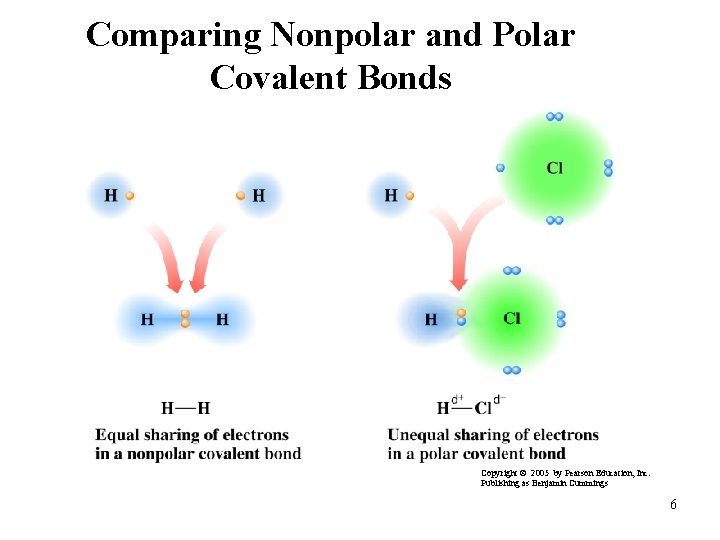
Comparing Nonpolar and Polar Covalent Bonds Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 6
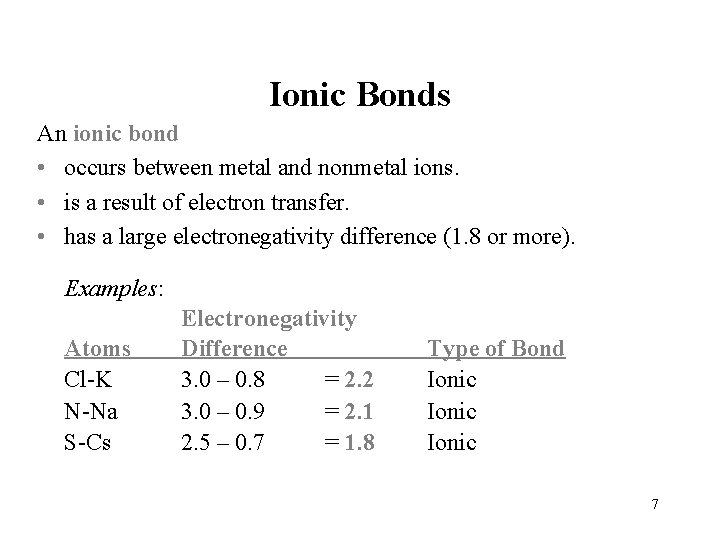
Ionic Bonds An ionic bond • occurs between metal and nonmetal ions. • is a result of electron transfer. • has a large electronegativity difference (1. 8 or more). Examples: Atoms Cl-K N-Na S-Cs Electronegativity Difference 3. 0 – 0. 8 = 2. 2 3. 0 – 0. 9 = 2. 1 2. 5 – 0. 7 = 1. 8 Type of Bond Ionic 7
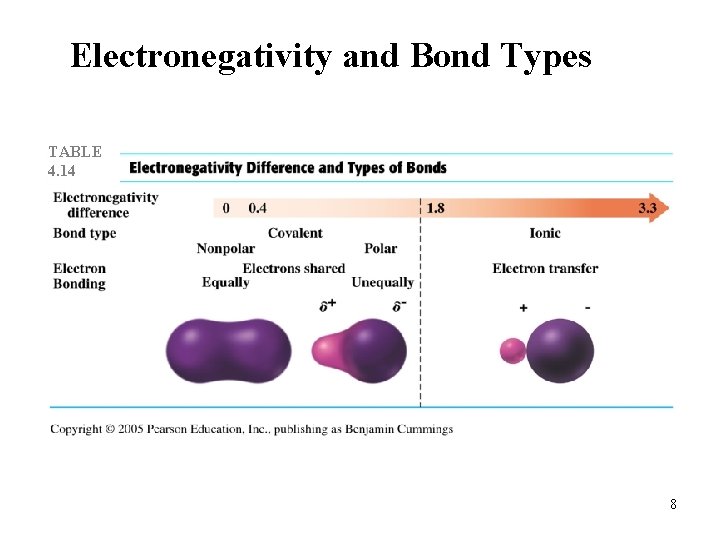
Electronegativity and Bond Types TABLE 4. 14 8
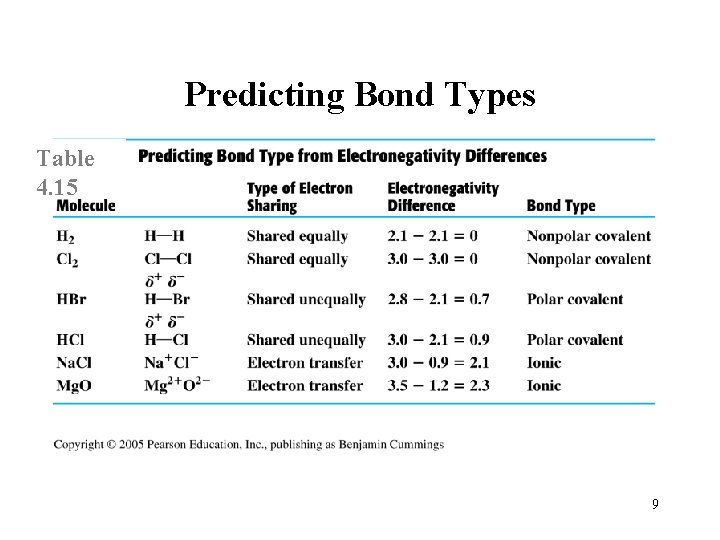
Predicting Bond Types Table 4. 15 9
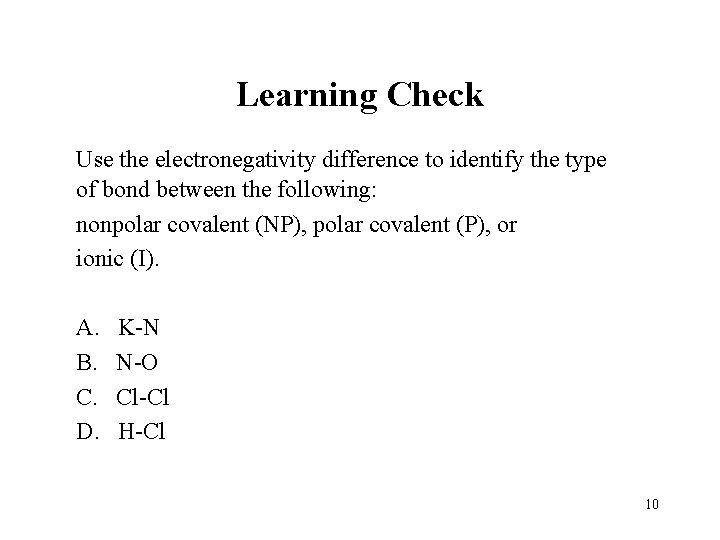
Learning Check Use the electronegativity difference to identify the type of bond between the following: nonpolar covalent (NP), polar covalent (P), or ionic (I). A. B. C. D. K-N N-O Cl-Cl H-Cl 10
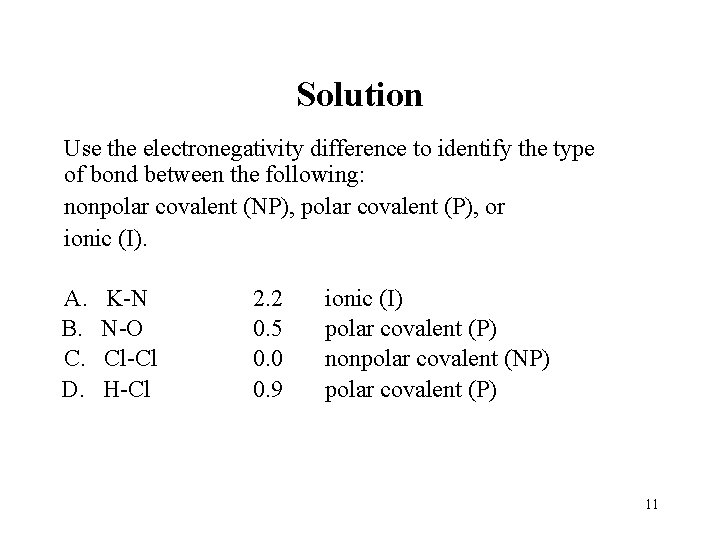
Solution Use the electronegativity difference to identify the type of bond between the following: nonpolar covalent (NP), polar covalent (P), or ionic (I). A. B. C. D. K-N N-O Cl-Cl H-Cl 2. 2 0. 5 0. 0 0. 9 ionic (I) polar covalent (P) nonpolar covalent (NP) polar covalent (P) 11
Bond energies
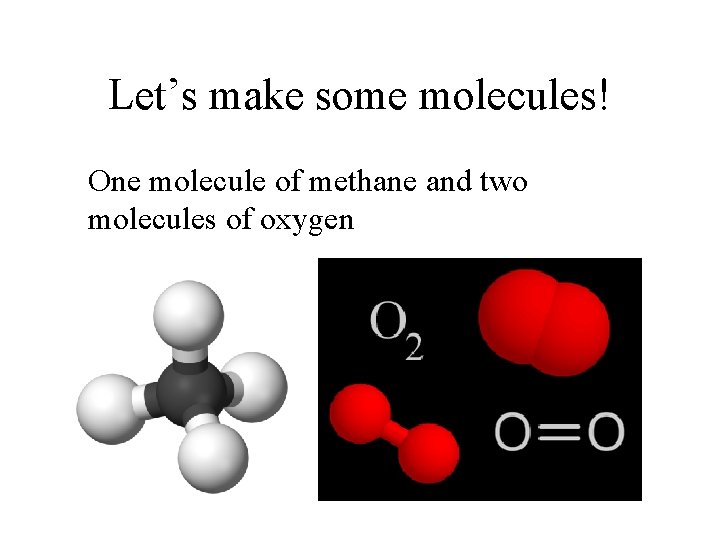
Let’s make some molecules! One molecule of methane and two molecules of oxygen
Combustion of methane?
Combustion of methane CH 4(g) + 2 O 2(g) 2 H 2 O(l) + CO 2(g)
Combustion of methane CH 4(g) + 2 O 2(g) 2 H 2 O(l) + CO 2(g) All reactions involve bond breaking and bond making as the atoms “swap partners”
Bond breaking - endothermic • Energy is always required to be inputted to break a bond. Bond breaking is always endothermic.

Bond making - exothermic • Energy is always released when a bond is formed. Bond making is always exothermic.
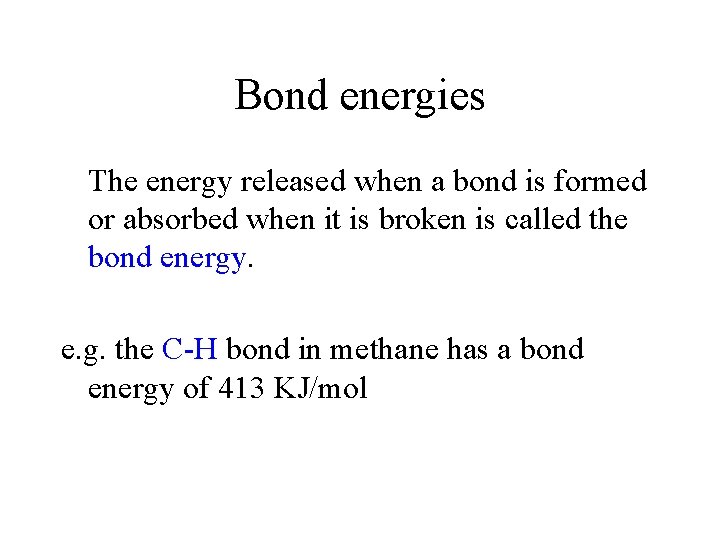
Bond energies The energy released when a bond is formed or absorbed when it is broken is called the bond energy. e. g. the C-H bond in methane has a bond energy of 413 KJ/mol
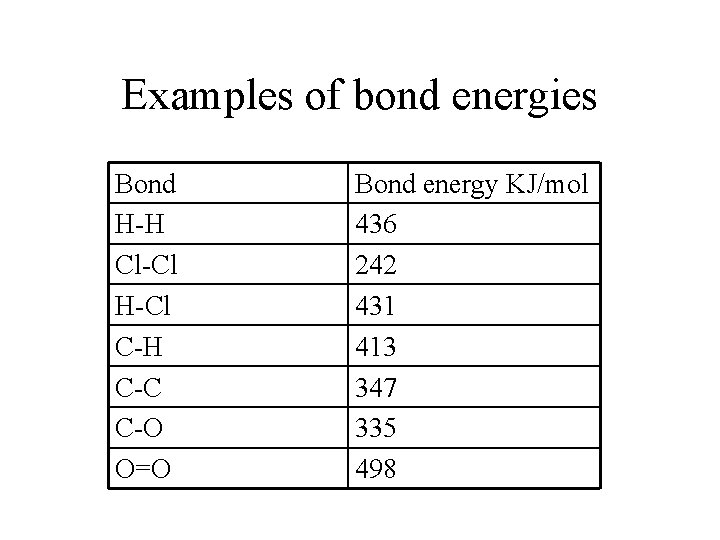
Examples of bond energies Bond H-H Cl-Cl H-Cl C-H C-C C-O O=O Bond energy KJ/mol 436 242 431 413 347 335 498
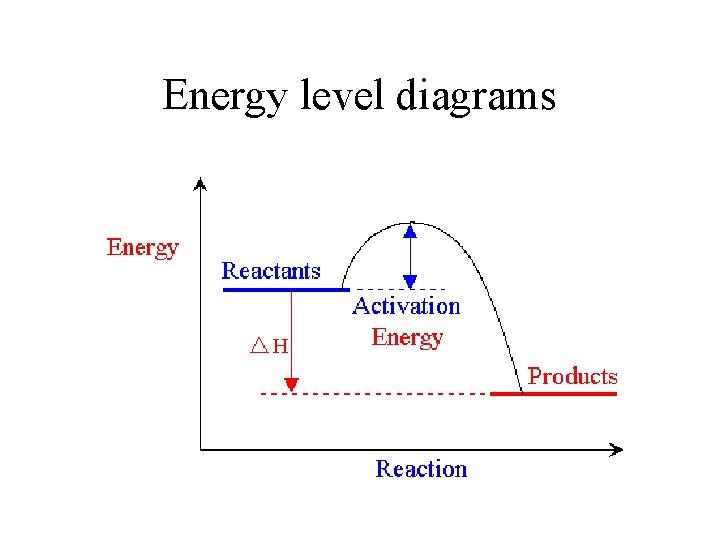
Energy level diagrams
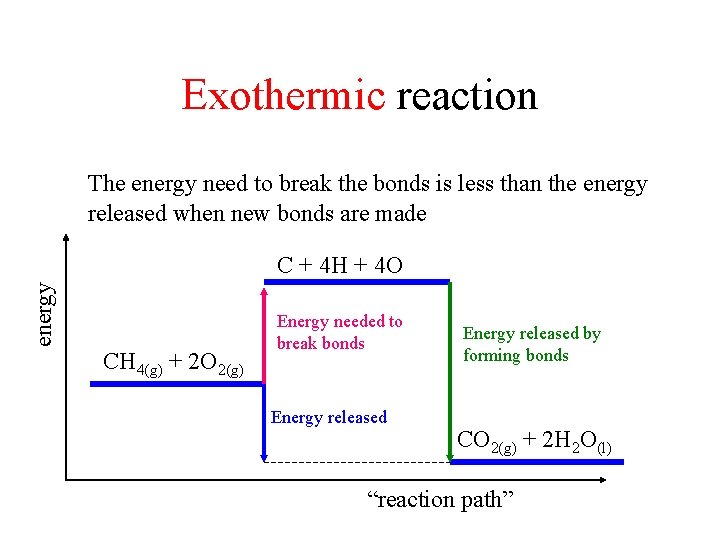
Exothermic reaction The energy need to break the bonds is less than the energy released when new bonds are made energy C + 4 H + 4 O CH 4(g) + 2 O 2(g) Energy needed to break bonds Energy released by forming bonds CO 2(g) + 2 H 2 O(l) “reaction path”
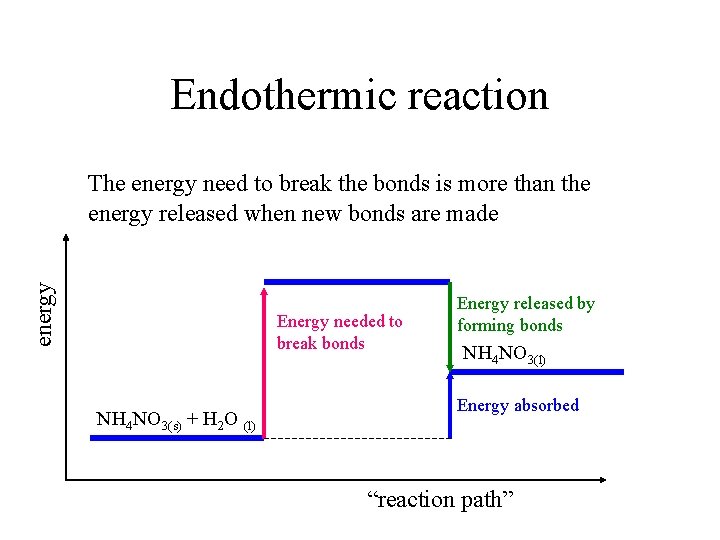
Endothermic reaction energy The energy need to break the bonds is more than the energy released when new bonds are made Energy needed to break bonds NH 4 NO 3(s) + H 2 O (l) Energy released by forming bonds NH 4 NO 3(l) Energy absorbed “reaction path”
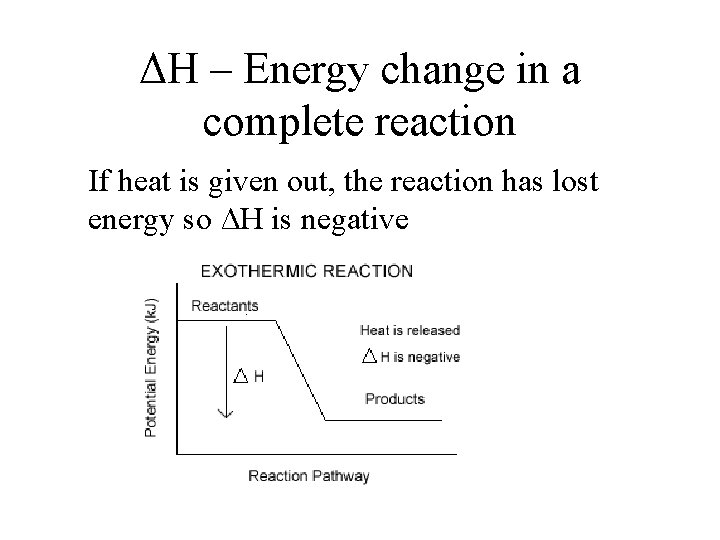
ΔH – Energy change in a complete reaction If heat is given out, the reaction has lost energy so ΔH is negative
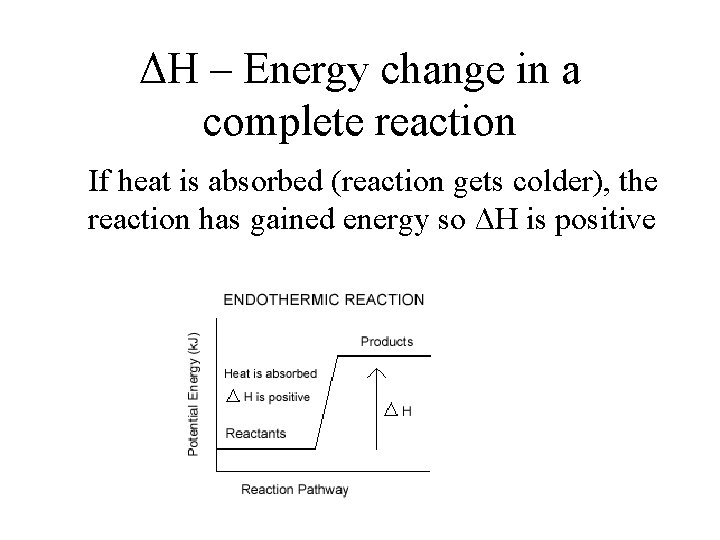
ΔH – Energy change in a complete reaction If heat is absorbed (reaction gets colder), the reaction has gained energy so ΔH is positive
Calculating ΔH CH 4(g) + 2 O 2(g) 2 H 2 O(l) + CO 2(g)
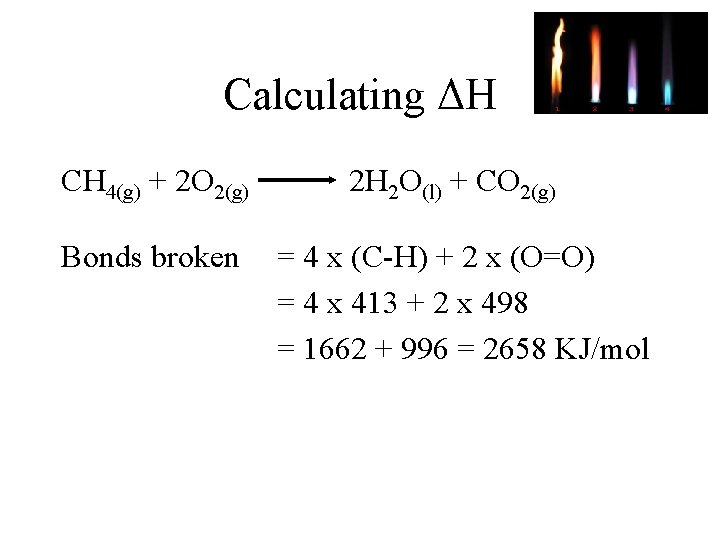
Calculating ΔH CH 4(g) + 2 O 2(g) Bonds broken 2 H 2 O(l) + CO 2(g) = 4 x (C-H) + 2 x (O=O) = 4 x 413 + 2 x 498 = 1662 + 996 = 2658 KJ/mol
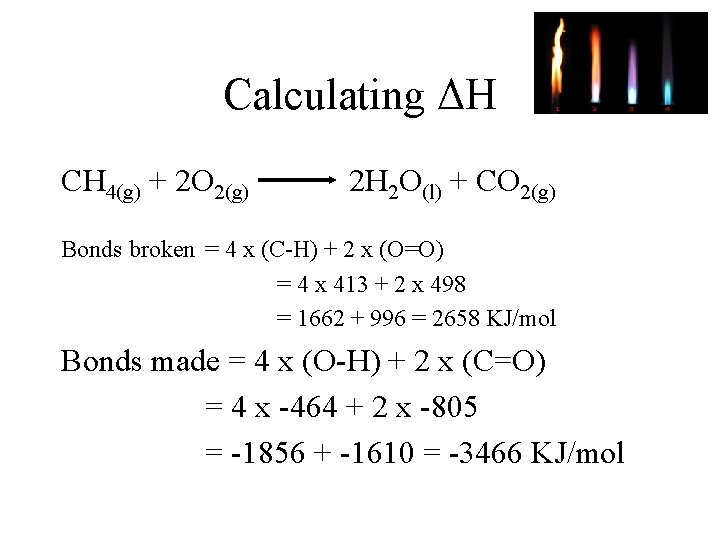
Calculating ΔH CH 4(g) + 2 O 2(g) 2 H 2 O(l) + CO 2(g) Bonds broken = 4 x (C-H) + 2 x (O=O) = 4 x 413 + 2 x 498 = 1662 + 996 = 2658 KJ/mol Bonds made = 4 x (O-H) + 2 x (C=O) = 4 x -464 + 2 x -805 = -1856 + -1610 = -3466 KJ/mol
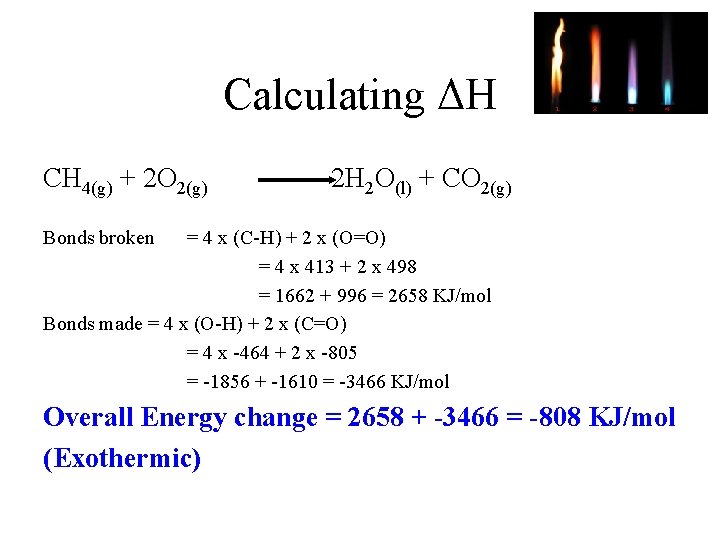
Calculating ΔH CH 4(g) + 2 O 2(g) 2 H 2 O(l) + CO 2(g) Bonds broken = 4 x (C-H) + 2 x (O=O) = 4 x 413 + 2 x 498 = 1662 + 996 = 2658 KJ/mol Bonds made = 4 x (O-H) + 2 x (C=O) = 4 x -464 + 2 x -805 = -1856 + -1610 = -3466 KJ/mol Overall Energy change = 2658 + -3466 = -808 KJ/mol (Exothermic)
Summary What do you need to know? • Breaking bonds is endothermic. Making bonds is exothermic. • How to recognize bond length and bond energy from the graphs. • How to compare two bonding graphs. • Be able to use bond energies to approximate DH.
- Slides: 30