Compounds and Mixtures We can divide all substances
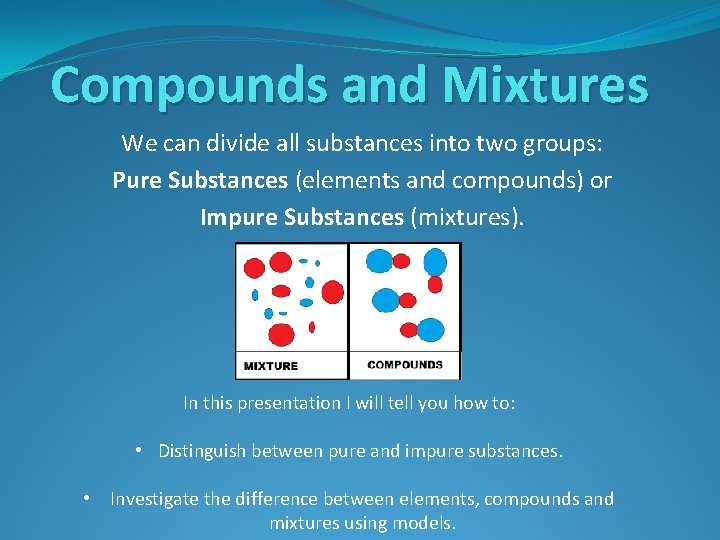
Compounds and Mixtures We can divide all substances into two groups: Pure Substances (elements and compounds) or Impure Substances (mixtures). In this presentation I will tell you how to: • Distinguish between pure and impure substances. • Investigate the difference between elements, compounds and mixtures using models.
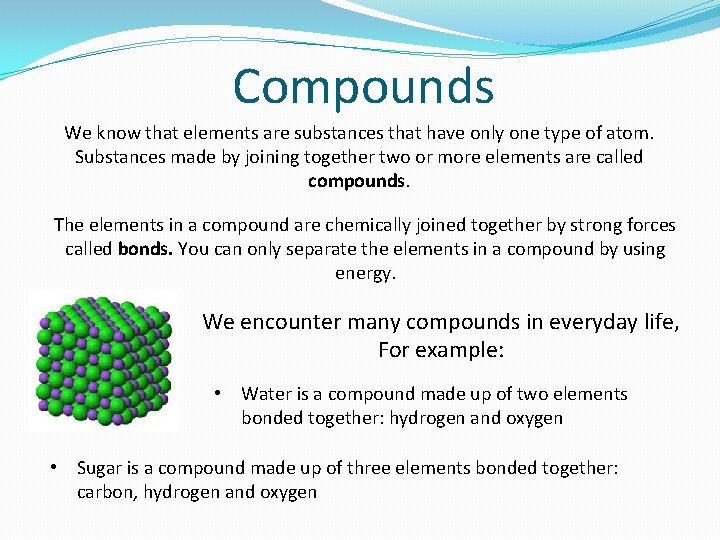
Compounds We know that elements are substances that have only one type of atom. Substances made by joining together two or more elements are called compounds. The elements in a compound are chemically joined together by strong forces called bonds. You can only separate the elements in a compound by using energy. We encounter many compounds in everyday life, For example: • Water is a compound made up of two elements bonded together: hydrogen and oxygen • Sugar is a compound made up of three elements bonded together: carbon, hydrogen and oxygen
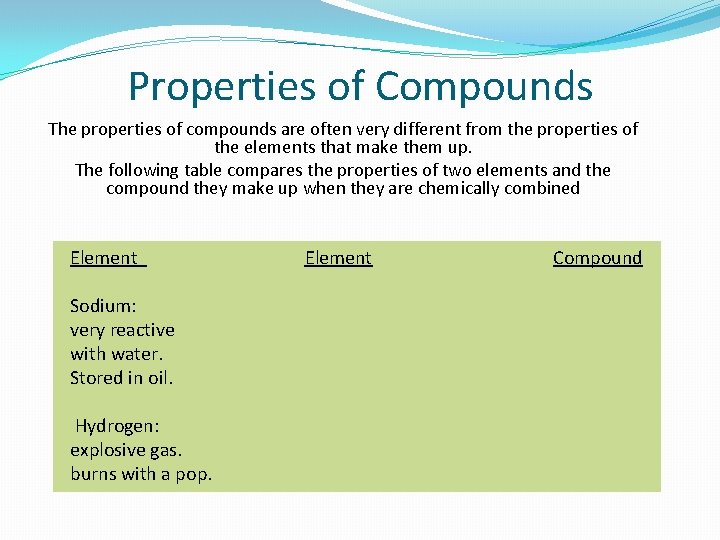
Properties of Compounds The properties of compounds are often very different from the properties of the elements that make them up. The following table compares the properties of two elements and the compound they make up when they are chemically combined Element Sodium: very reactive with water. Stored in oil. Hydrogen: explosive gas. burns with a pop. Element Compound
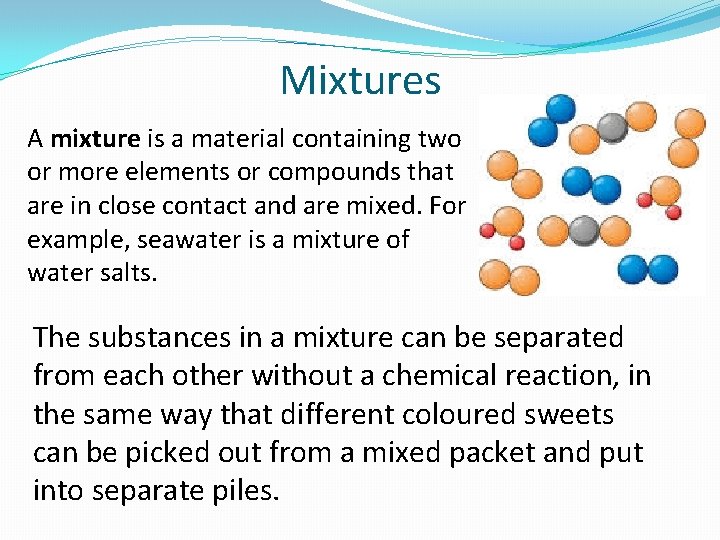
Mixtures A mixture is a material containing two or more elements or compounds that are in close contact and are mixed. For example, seawater is a mixture of water salts. The substances in a mixture can be separated from each other without a chemical reaction, in the same way that different coloured sweets can be picked out from a mixed packet and put into separate piles.
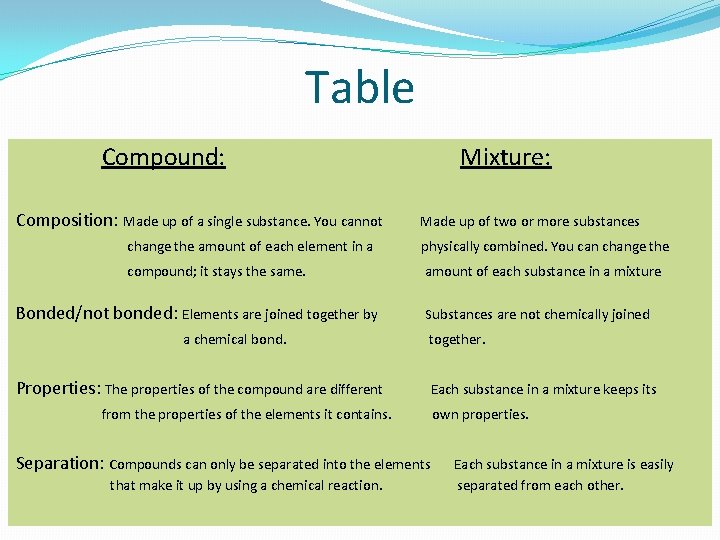
Table Compound: Composition: Made up of a single substance. You cannot Mixture: Made up of two or more substances change the amount of each element in a physically combined. You can change the compound; it stays the same. amount of each substance in a mixture Bonded/not bonded: Elements are joined together by a chemical bond. Substances are not chemically joined together. Properties: The properties of the compound are different from the properties of the elements it contains. Separation: Compounds can only be separated into the elements that make it up by using a chemical reaction. Each substance in a mixture keeps its own properties. Each substance in a mixture is easily separated from each other.

Pure vs Impure Substances Pure • A Pure element or compound contains only one substance, with no other substances mixed in. Examples Impure • Impure materials may be mixtures of elements, mixtures of compounds, or mixtures of elements and compounds. • Pure: tin, sulphur, diamond, water, pure sugar (sucrose), table salt (sodium chloride) and baking soda (sodium bicarbonate). • Impure: steel, silicon, carbonated water, copper, sterling silver, 14 karat gold and platinum.

END Thank you for watching. Made by: Alex Mc. Gann
- Slides: 7