Compounds and formulas Chemical Reactions Balancing Equations Acids
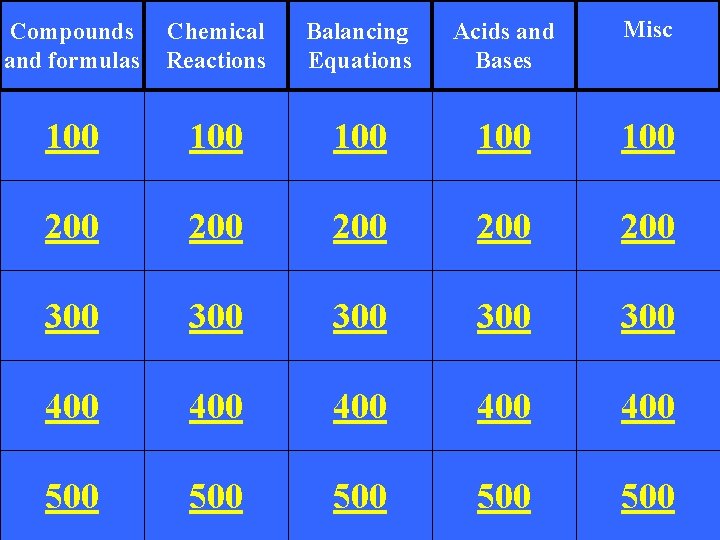
Compounds and formulas Chemical Reactions Balancing Equations Acids and Bases Misc 100 100 100 200 200 200 300 300 300 400 400 400 500 500 500

Ionic compounds are composed of this.

What are metals and non-metals?

The prefixes of naming covalent compounds from 1 -6.

What are mono, di, tri, tetra, penta and hexa?

aluminum sulfate

What is Al 2(SO 4)3?

Name (NH 4)2 SO 4

Ammonium sulfate

2 of the 3 Criteria for Molecular Compounds.

What are: composed of pairs of electrons being shared; attraction is called a covalent bond; two non-metals.

Exothermic.

What is an energy releasing reaction? (i. e. explosions)

In the law of conservation of energy: energy can be converted from one form to another but this stays constant.

What is the total quantity of energy?

Three of the five types of reactions.

What is Synthesis; Decomposition; Single Displacement; Combustion and /or Double Displacement?
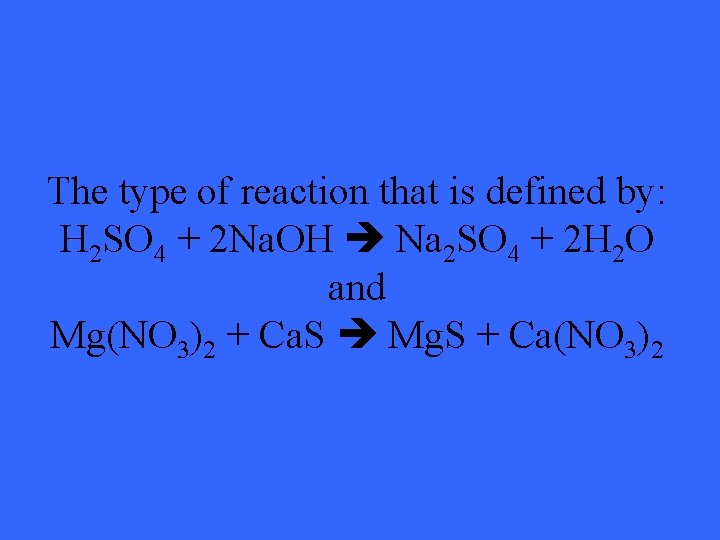
The type of reaction that is defined by: H 2 SO 4 + 2 Na. OH Na 2 SO 4 + 2 H 2 O and Mg(NO 3)2 + Ca. S Mg. S + Ca(NO 3)2

What is double displacement?
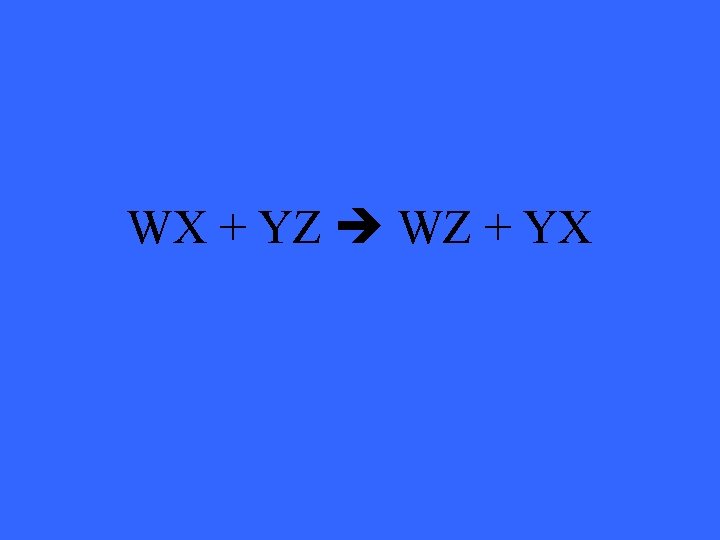
WX + YZ WZ + YX

What is a double displacement reaction?

The total mass of the reactants = the total mass of the products.

What is the law of conservation of mass?
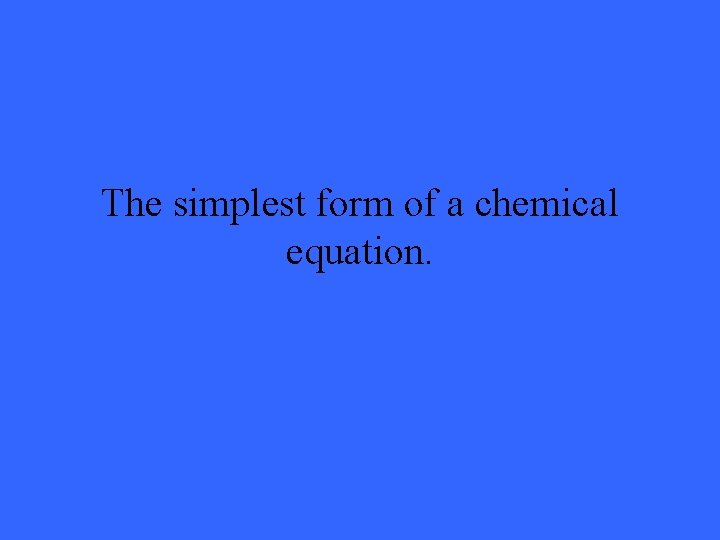
The simplest form of a chemical equation.
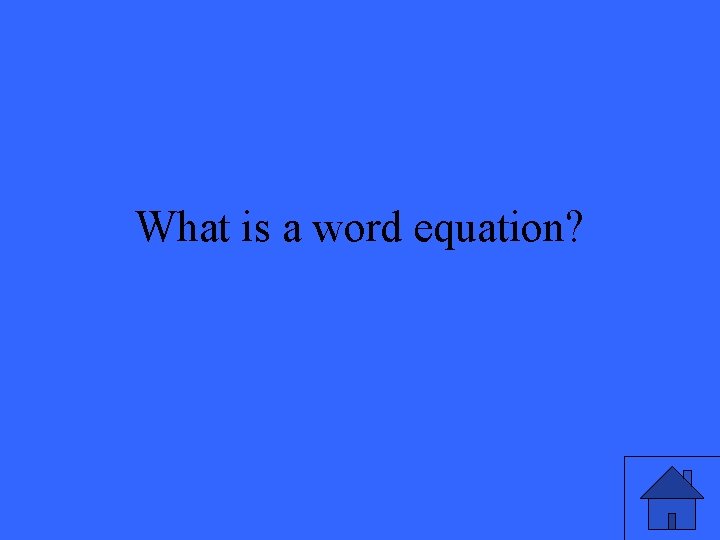
What is a word equation?
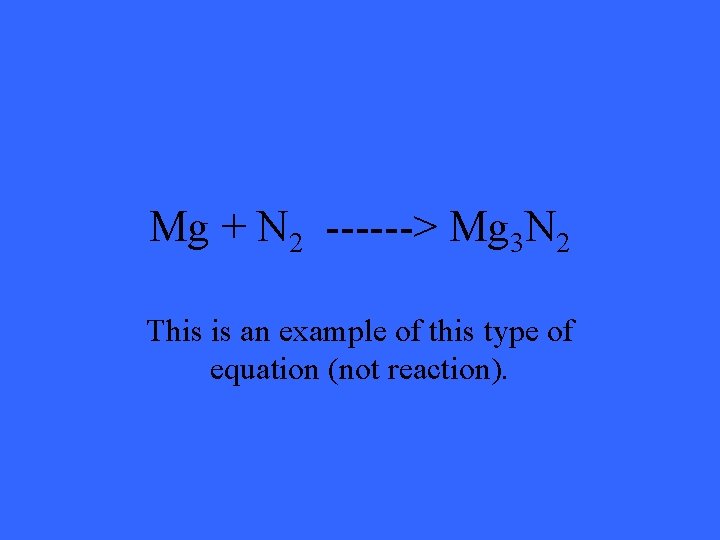
Mg + N 2 ------> Mg 3 N 2 This is an example of this type of equation (not reaction).
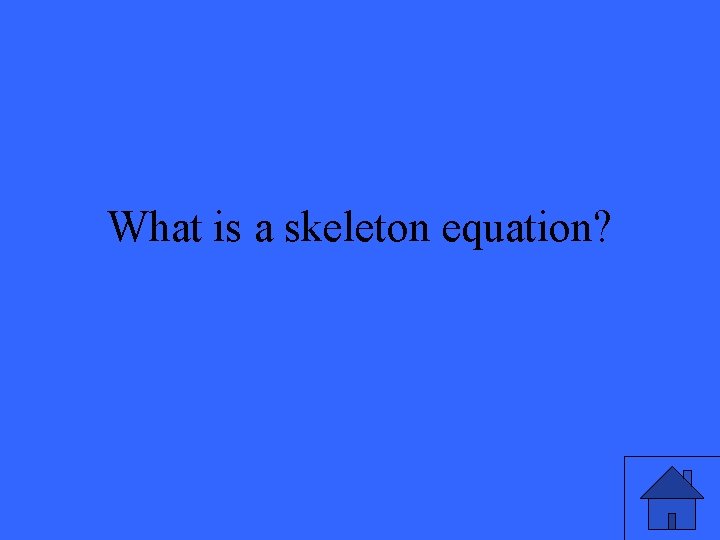
What is a skeleton equation?
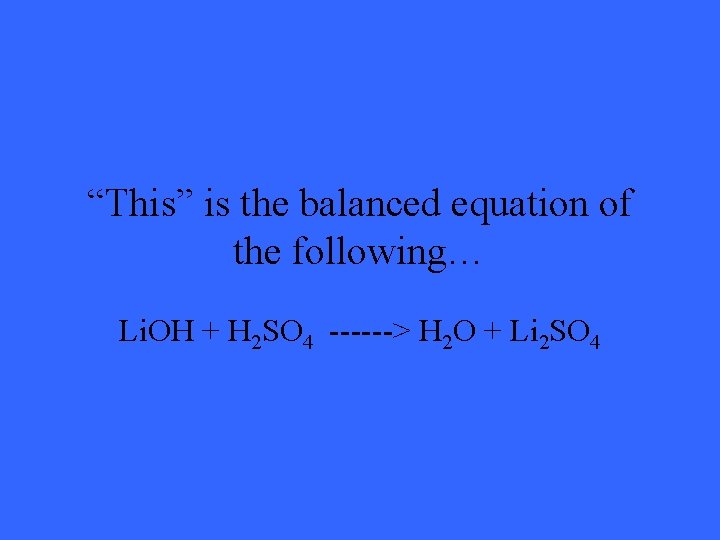
“This” is the balanced equation of the following… Li. OH + H 2 SO 4 ------> H 2 O + Li 2 SO 4
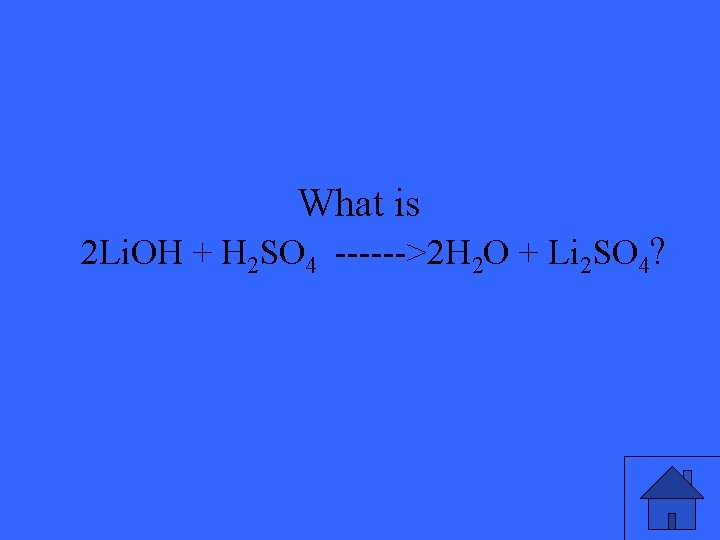
What is 2 Li. OH + H 2 SO 4 ------>2 H 2 O + Li 2 SO 4?
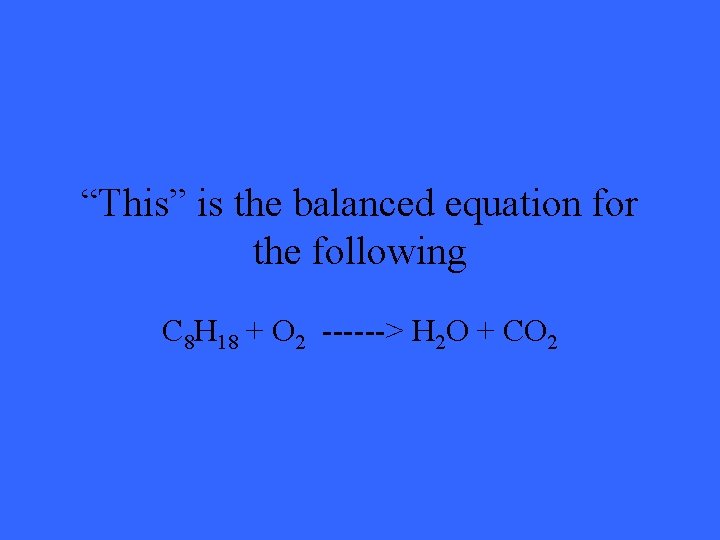
“This” is the balanced equation for the following C 8 H 18 + O 2 ------> H 2 O + CO 2
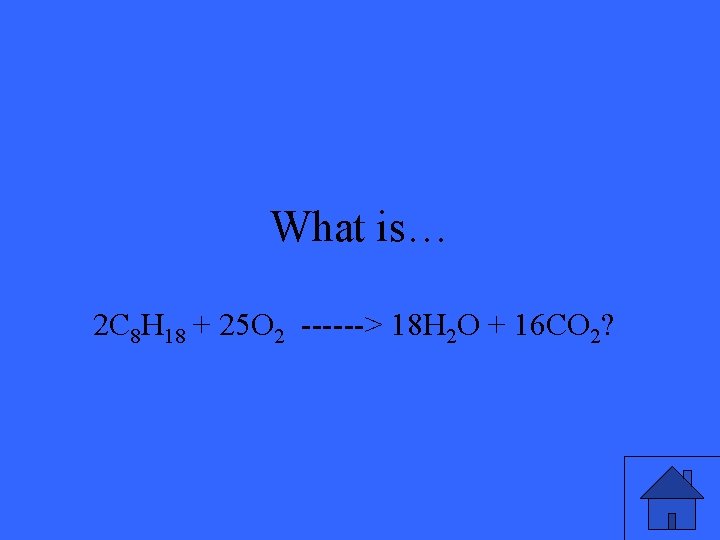
What is… 2 C 8 H 18 + 25 O 2 ------> 18 H 2 O + 16 CO 2?

The measuring scale of acids and bases.

What is p. H?

The reaction of an acid and a base.

What is a neutralization reaction?

This turns colorless in acidic solutions and pinkish in basic solutions.

What is phenolphthalein?

Type of substance you are attempting to name if it either: 1. Contains hydrogen and a non-metallic element and you use the prefix “hydro” THEN add suffix “ic” to non-metallic element or 2. Contains a polyatomic ion with an oxygen atom ending in “ate” and you start with name of the element in the polyatomic ion (not oxygen) THEN add suffix “ic”

What is an acid?

This substance will turn red litmus paper blue, feels slippery and will conduct electricity in an aqueous solution.

What is a base?

Outer portion of an atom, with electrons called valence electrons.

What is the valence shell?

The word equation for a typical combustion reaction.

What is Hydrocarbon + oxygen carbon dioxide + water

Different oxides of sulfur (SO 2 and SO 3) and nitrogen (NO and NO 2) are referred to as SOx and NOx. When they are burned in air they respectfully form what substances?

What are sulfuric acid and nitric acid?

Name of process in which calcium carbonate (limestone) reacts with acids in a lake.

What is liming?

As the acidity within a lake approaches 6, insects and other aquatic animals begin to die. These two things occur next.

What is as it approaches 5, plants and micro-organisms begin to die. Below 5 and all life in a lake or pond is gone and the water appears crystal clear.
- Slides: 51