Compounds and Bonding Part 3 Covalent Bonds Review

Compounds and Bonding Part 3: Covalent Bonds
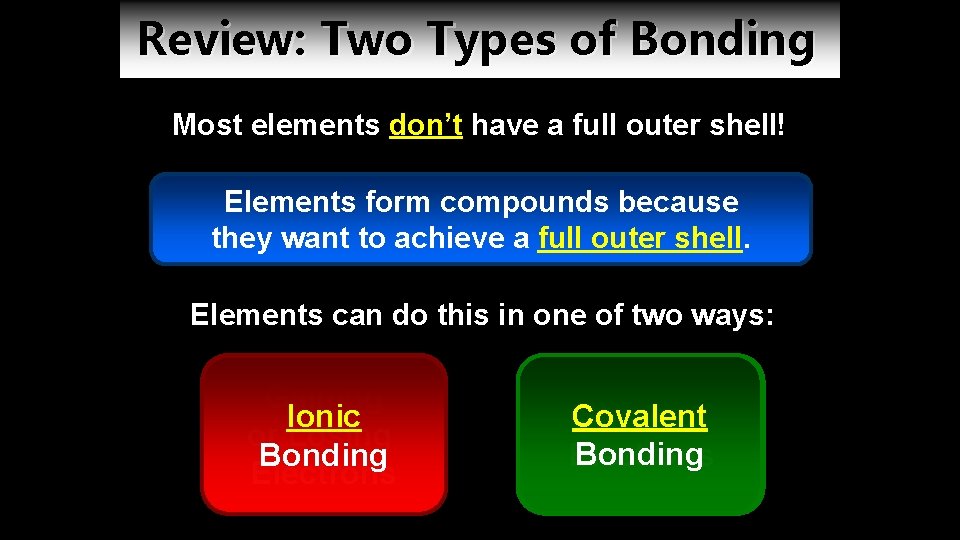
Review: Two Types of Bonding Most elements don’t have a full outer shell! Elements form compounds because they want to achieve a full outer shell. Elements can do this in one of two ways: Gaining Ionic or Losing Bonding Electrons Covalent Sharing Bonding Electrons
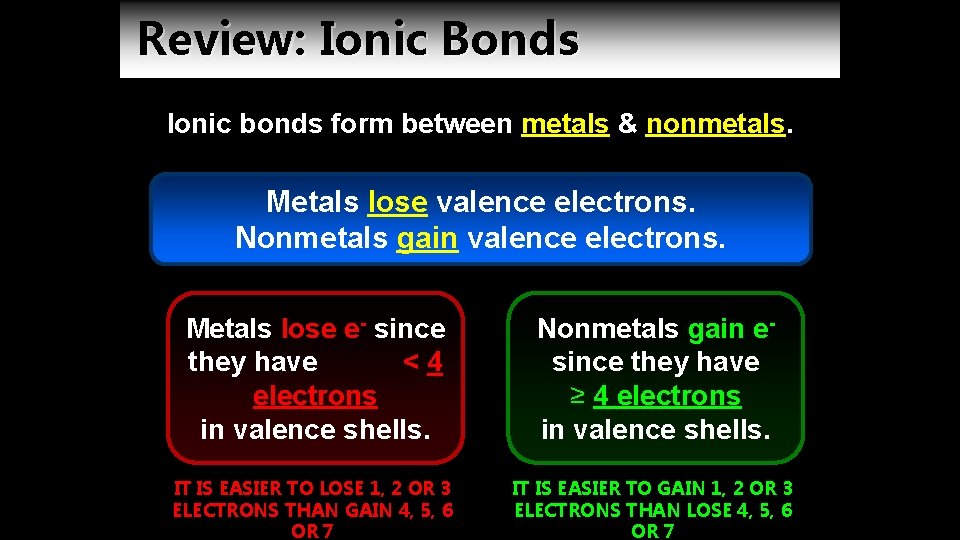
Review: Ionic Bonds Ionic bonds form between metals & nonmetals. Metals lose valence electrons. Nonmetals gain valence electrons. Metals lose e- since they have <4 electrons in valence shells. Nonmetals gain esince they have ≥ 4 electrons in valence shells. IT IS EASIER TO LOSE 1, 2 OR 3 ELECTRONS THAN GAIN 4, 5, 6 OR 7 IT IS EASIER TO GAIN 1, 2 OR 3 ELECTRONS THAN LOSE 4, 5, 6 OR 7
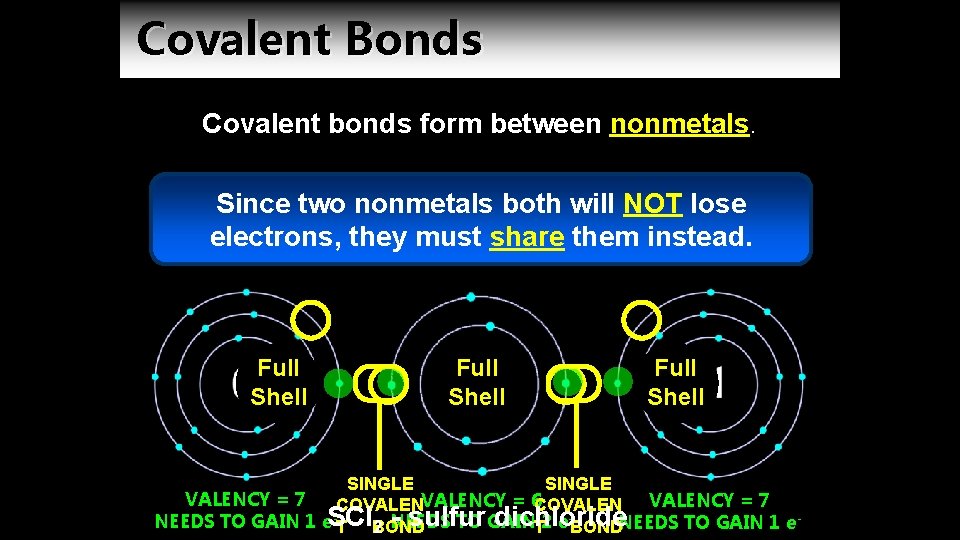
Covalent Bonds Covalent bonds form between nonmetals. Since two nonmetals both will NOT lose electrons, they must share them instead. Full Shell SINGLE VALENCY = 7 COVALENCY = 6 COVALEN - sulfur dichloride NEEDS TO GAIN 1 e. SCl TO GAIN T BOND T 2 e BONDNEEDS TO GAIN 1 e 2 NEEDS

Covalent Bonds Covalent bonds form between nonmetals. Since two nonmetals both will NOT lose electrons, they must share them instead. Atoms can share more than 1 pair of electrons: Double Two Pairs Bond Triple Three Bond Pairs O C O N N CO 2 - CARBON DIOXIDE N 2 - NITROGEN GAS
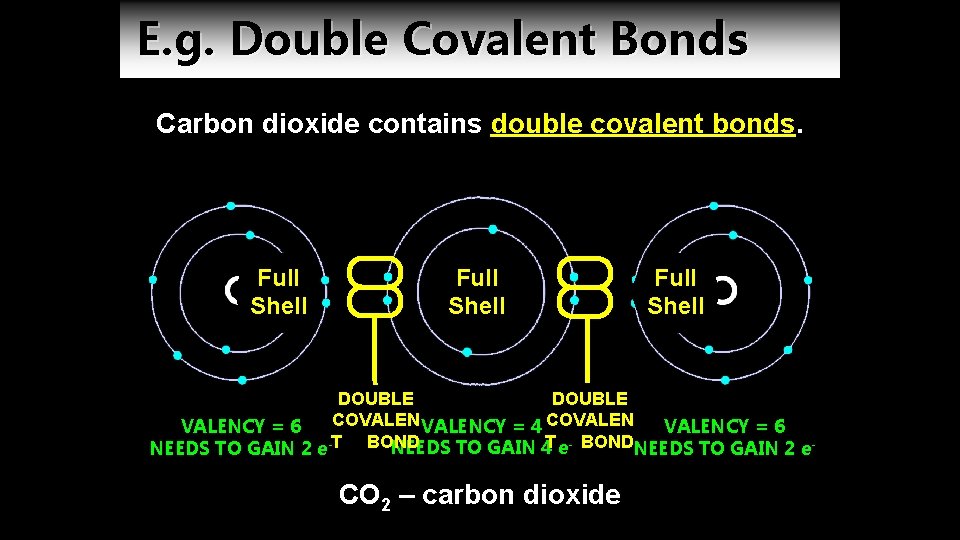
E. g. Double Covalent Bonds Carbon dioxide contains double covalent bonds. Full Shell DOUBLE COVALENCY = 4 COVALENCY = 6 T BOND NEEDS TO GAIN 4 e. NEEDS TO GAIN 2 e- CO 2 – carbon dioxide
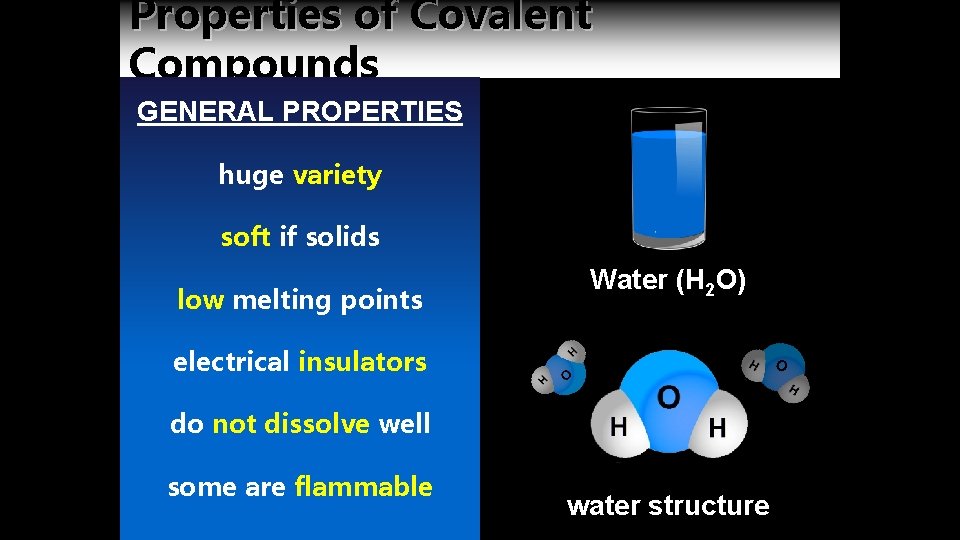
Properties of Covalent Compounds GENERAL PROPERTIES huge variety soft if solids low melting points Water (H 2 O) electrical insulators do not dissolve well some are flammable water structure
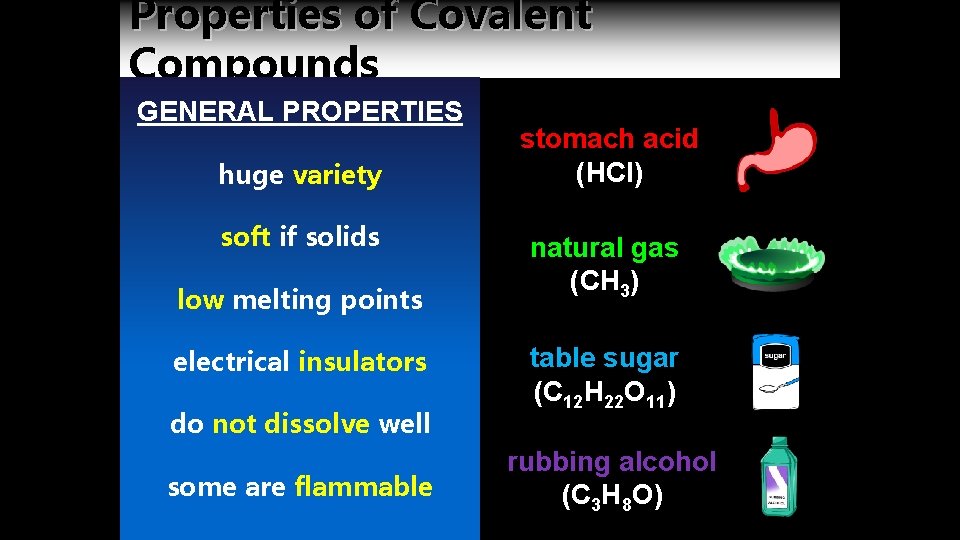
Properties of Covalent Compounds GENERAL PROPERTIES huge variety soft if solids low melting points electrical insulators do not dissolve well some are flammable stomach acid (HCl) natural gas (CH 3) table sugar (C 12 H 22 O 11) rubbing alcohol (C 3 H 8 O)
- Slides: 8