Compounding Technique Rubber compounding What is rubber compounding

Compounding Technique

Rubber compounding What is rubber compounding? Why we are doing? How can we do it successfully?

Definition of Rubber Compounding • is the art and science of selecting and combining elastomers and additives to obtain an intimate mixing that will develop the necessary physical and chemical properties for a finished product.

Objective of Rubber Compounding. 1 To secure certain properties in the finished product to satisfy service requirements. . 2 To attain processing characteristics necessary for efficient utilization of available equipment. . 3 To achieve the desirable properties and processability at lowest possible cost.

To be Sucessful in Compounding • Must understand the properties and function of hundreds of elastomers and rubber chemicals • Must also have intimate knowledge of the equipment used for mixing, extrusion, calendering, molding and vulcanization.

Procedure for Compound Development • . 1 Set specific objectives (properties, price, etc. (. • . 2 Select base elastomer(s. ( • . 3 Study test data of existing compounds. • . 4 Survey compound formulations and properties data presented by material suppliers in their literature. • . 5 Choose a starting formulation.

To be continued • . 6 Develop compounds in laboratory to meet objectives. • . 7 Estimate cost of compound selected for further evaluation. • . 8 Evaluate processability of compound in factory. • . 9 Use compound to make a product sample • . 10 Test product sample against performance specification.

Classification of Compounding Ingredients • . 1 Elastomers • . 2 Vulcanizing Agents (Curatives( • . 3 Accelerators • . 4 Activators and Retarders • . 5 Antidegradants(Antioxidants, Antiozonants, Protective Waxes( • . 6 Processing Aids(Peptizers, Lubricants, Release Agents (

To be continued • . 7 Fillers (Carbon Blacks, Non-black Materials( • . 8 Plasticizers, Softeners, and Tackifiers • . 9 Color Pigments • . 10 Special Purpose Materials(Blowing Agents, Reodorants, etc(,

Requirements of Rubber Compound for Good Processing • . 1 Uniform plasticity and recovery. • . 2 Uniform scorch rate. • . 3 Uniform rate of cure.

Vulcanizing Agents • To cause chemical reaction resulting in crosslinking of elastomer molecules. • Sulfur is by far the most widely used.

VULCANIZING AGENTS • TYPE • Sulfur or Sulfurbearing Materials COMMON USE Natural Rubber, Isoprene, SBR, Buty 1, Butadiene, EPDM, Nitrile, Norsorex • Organic Peroxides Urethane, Silicone, Chlorinated Polyethylene, Crosslinked Polyethylene, Vamac, Vynathene, PVC/ Nitrile

To be continued • TYPE • Metallic Oxides • Organic Amines • Phenolic Resins COMMON USE Neoprene, Hypalon, Thiokol Acrylic, Fluorocarbon, Epichlorohydrin, Vamac Butyl

ACCELERATORS • Use to reduce vulcanization time, or cure time by increasing the speed of vulcanization • Most are organic substance containing both nitrogen and sulfur(Today( • Inorganic accelerator was widelyused years ago (litharge, lime, and magnesia(
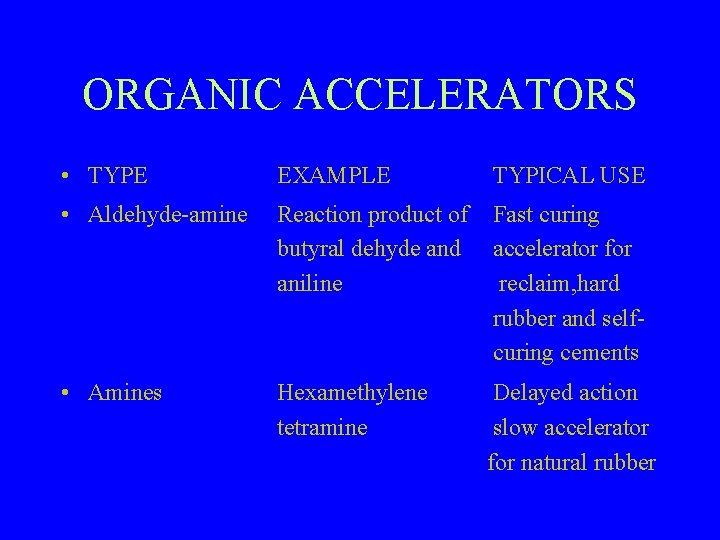
ORGANIC ACCELERATORS • TYPE EXAMPLE TYPICAL USE • Aldehyde-amine Reaction product of butyral dehyde and aniline Fast curing accelerator for reclaim, hard rubber and selfcuring cements • Amines Hexamethylene tetramine Delayed action slow accelerator for natural rubber

To be continued • TYPE • Guanidines EXAMPLE Diphenyl guanidine (DPG) TYPICAL USE Secondary accelerator to activate thiazole type accelerator • Thioureas Ethylene thiourea Fast curing accelerator (ETU) for Neoprene, Hypalon and Epichlorohydrin

To be continued • TYPE • Thiazoles • Thiurams EXAMPLE TYPICAL USE Benzothiazyldisulfide Safe-processing (MBTS) moderately fast curing accelerator for natural rubber, Isoprene, SBR, Nitrile, Butyl and EPDM Tetramethylthiuram Fast curing sulfurdisulfide (TMTD) bearing accelerator for SBR, Nitrile, Butyl and EPDM

To be continued • TYPE EXAMPLE • Sulfenamides N-cyclohexyl-2 benzothiazylsulfenamide (CBS) TYPICAL USE Safe-processing, delayed action accelerator for natural rubber, SBR and Nitrile • Dithiocarbamates Zinc dimethyl Fast curing accelerator • Xanthates Dibutylxanthogen Fast curing, low disulfide temperature accelerator for natural rubber and SBR

Activators and Retarders • Activators - used to activate the accelerator and improve its effectiveness (Zn. O, stearic acid, litharge, magnesia, and amine) - attain good crosslink efficiency • Retarders - used to reduce the scorchness (phthalic anhydride, salicylic acid and sodium acetate (

Antidegradants • To retard the deterioration of rubber compounds initiated by - oxygen, ozone - heat, light - metal catalyst and - mechanical flexing

PROCESSING AIDS • To facilitate processing operation such as - Mixing - Calendering - Extrusion and - Molding
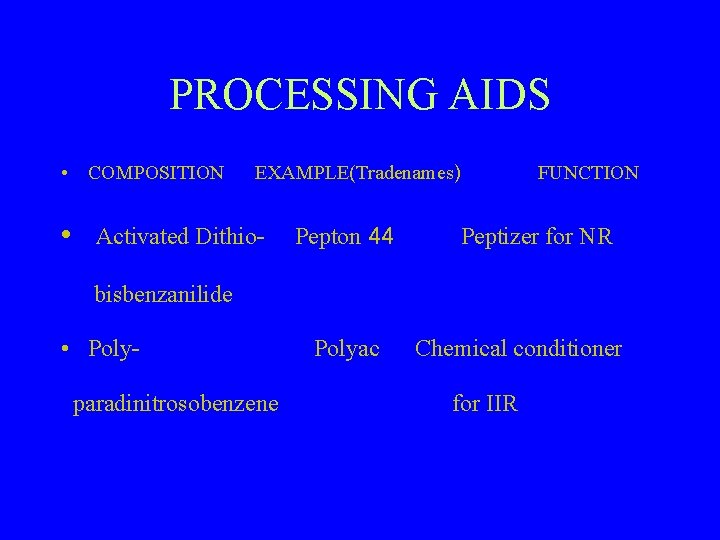
PROCESSING AIDS • COMPOSITION • EXAMPLE(Tradenames) Activated Dithio- Pepton 44 FUNCTION Peptizer for NR bisbenzanilide • Polyparadinitrosobenzene Polyac Chemical conditioner for IIR

To be continued • COMPOSITION EXAMPLE(Tradenames) • Xylyl mercaptans RPA 3 FUNCTION Peptizer for NR, IR, SBR and NBR. Stabilizer for cement viscosity • Low-molecularweight polyethylene A-C Polyethylene 617 A Release agent, lubricant • Calcium oxide Desi. Cal P Dessiccant

To be continued • COMPOSITION • Aliphaticnaphthenicaromatic resins • Paraffin Wax • Polyethylene glycol • Petroleum hydrocarbon EXAMPLE(Tradenames) Strucktol 60 NS FUNCTION Homogenizing agent for all elastomers Numerous Release agent, lubricant Carbowax PEG 3350 Activator for silica lubricant Petrolatum Release agent, lubricant

Fillers • To reinforce physical properties • To reduce cost • Devided into two types(Reinforcing and Extending( • Selection of reinforcing filler is the third most important task in compounding(next to elastomer and cure system(

Types of Fillers • Reinforcing Type Carbon Black (listed in order of increasing particle size) Non-Black N 220 (ISAF) N 330 (HAF) N 550 (FEF) N 762 (SRF-LM) N 990 (MT ( - Silica - Zinc Oxide - Magnesium Carbonate - Aluminum Silicate - Sodium Aluminosilicate - Magnesium Silicate

Types of Fillers (continued( Extending Type - Calcium Carbonate - Barium Sulfate - Aluminum Trihydrate - Talc

Hardness NR has hardness itself (no filler) 35 -40 IRHD • ISAF 1. 7 phr increases 1 IRHD • HAF 1. 9 phr increases 1 IRHD • Hisil 233 2 phr increases 1 IRHD • Hard clay 5 phr increases 1 IRHD • Soft clay 7. 7 phr increases 1 IRHD • Whiting Ca. CO 3 6. 4 phr increases 1 IRHD 2 phr decreases 1 IRHD • Oil

Particles Size • Play a major role in the tensile strength small particle size highest tensile strength at optimum loading • Fine fillers is difficult to process (need more energy for their dispersion into the elastomer( • Effects Mooney scorch small particle size the scorch resistance

PLASTICIZERS, SOFTENERS, AND TACKIFIERS • Objective for Using - Aid mixing, - Modify viscosity, - Produce tack, - Provide flexibility at low temperature

Selection of Plasticizers • • The important criteria are: Compatibility Efficiency Cost Example: Aromatic type oil is not compatibe with NR, Isoprene, IIR, EPDM Paraffinic type oil is not compatible with SBR, butadiene, NBR, CR

PLASTICIZERS, SOFTENERS, AND TACKIFIERS • CATEGORY OF MATERIAL • Petroleum Oils -Aromatic - Paraffinic - Naphthenic FUNCTION Plasticizer, Softener • Ester Plasticizers - Dioctyl phthalate - Dioctyl sebacate - Tributoxyethyl phospate Low temperature - Di (butoxyethyl) formal plasticizers - Triglycol ester of vegetable oil

To be continued • CATEGORY OF MATERIAL FUNCTION • Vulcanized Vegetable Oils Extender, Plasticizer • Asphaltic Hydrocarbon Extender, Plasticizer • Pine Tar Plasticizer, Tackifier • Resins -Coumarone-indene - Petroleum - Phenolic Tackifier, Plasticizer • Polymeric esters Extender, Plasticizer • Rosins - Hydrogenated rosin Tackifier

Special Purpose Materials • Not require in the majority of rubber compound • Used for specific purpose Example : Blowing agents - Reodorants - Adhesion promotors - Flame retardant - Fungicide - UV light absorbers
- Slides: 34