Compound Naming Basics Chemical compounds have unique names

Compound Naming Basics • Chemical compounds have unique names because they have unique chemical properties. • Binary compounds are made of only 2 elements • The cation (+ ion) is listed first. • The anion (- ion) is listed second. • The ending of the anion is changed to –ide • i. e. sodium + chlorine sodium chloride. • Try potassium + sulfur _______ potassium sulfide • Try magnesium + oxygen _____ magnesium oxide
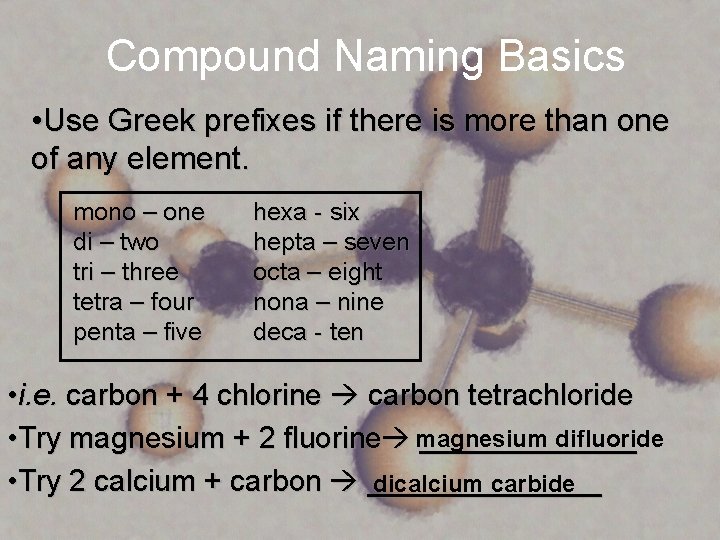
Compound Naming Basics • Use Greek prefixes if there is more than one of any element. mono – one di – two tri – three tetra – four penta – five hexa - six hepta – seven octa – eight nona – nine deca - ten • i. e. carbon + 4 chlorine carbon tetrachloride difluoride • Try magnesium + 2 fluorine magnesium _______ • Try 2 calcium + carbon _______ dicalcium carbide
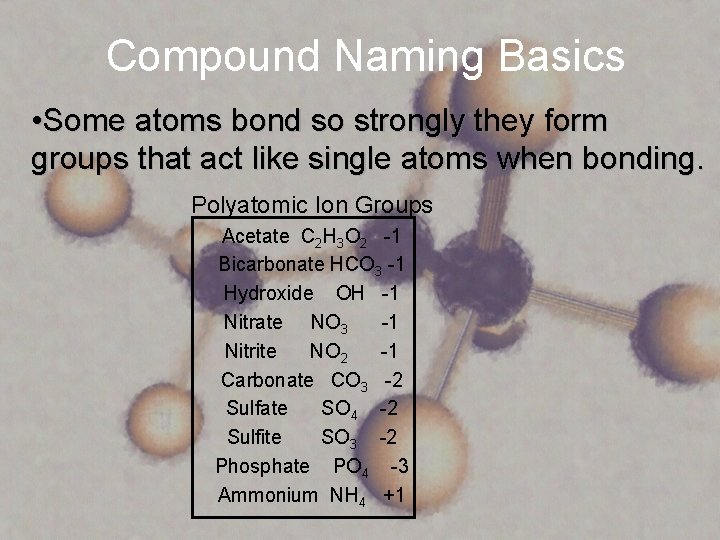
Compound Naming Basics • Some atoms bond so strongly they form groups that act like single atoms when bonding. Polyatomic Ion Groups Acetate C 2 H 3 O 2 -1 Bicarbonate HCO 3 -1 Hydroxide OH -1 Nitrate NO 3 -1 Nitrite NO 2 -1 Carbonate CO 3 -2 Sulfate SO 4 -2 Sulfite SO 3 -2 Phosphate PO 4 -3 Ammonium NH 4 +1
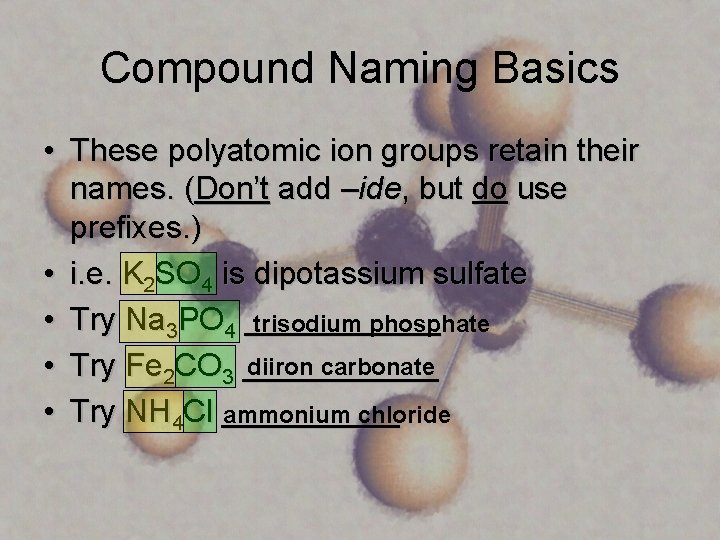
Compound Naming Basics • These polyatomic ion groups retain their names. (Don’t add –ide, but do use prefixes. ) • i. e. K 2 SO 4 is dipotassium sulfate • Try Na 3 PO 4 ______ trisodium phosphate diiron carbonate • Try Fe 2 CO 3 ______ • Try NH 4 Cl _____ ammonium chloride
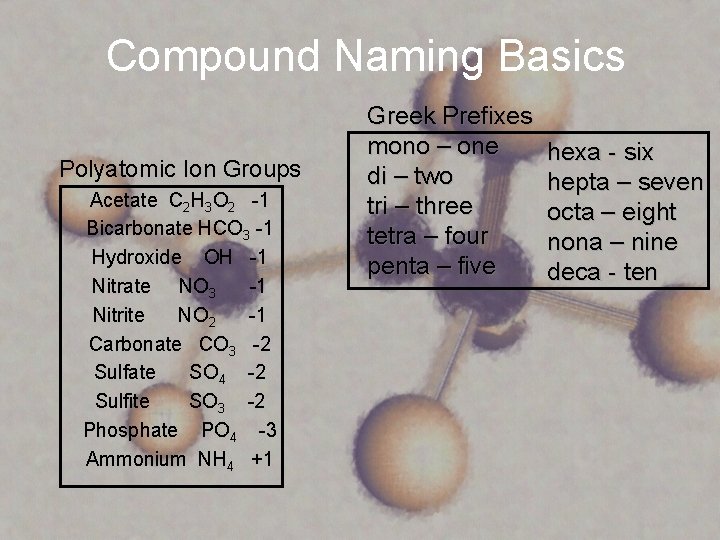
Compound Naming Basics Polyatomic Ion Groups Acetate C 2 H 3 O 2 -1 Bicarbonate HCO 3 -1 Hydroxide OH -1 Nitrate NO 3 -1 Nitrite NO 2 -1 Carbonate CO 3 -2 Sulfate SO 4 -2 Sulfite SO 3 -2 Phosphate PO 4 -3 Ammonium NH 4 +1 Greek Prefixes mono – one di – two tri – three tetra – four penta – five hexa - six hepta – seven octa – eight nona – nine deca - ten
- Slides: 5