Composition of Matter PROPERTIES OF MATTER What is
























- Slides: 32

Composition of Matter

PROPERTIES OF MATTER • What is matter? Anything that has mass and takes up space • What is mass? The amount of matter an object contains

SUBSTANCES • A substance is matter that has a definite and uniform composition One type of “stuff”

TYPES OF SUBSTANCES • There are two different types of substances: One type of “stuff”

ELEMENTS • An element is the simplest forms of matter. Carbon is an element Elements are made of atoms Diamonds are made of atoms of the element Carbon Foil is made of the atoms of the element Aluminum. • Each element is represented by a chemical symbol.

Atoms are matter • Carbon is an element • Elements are made of atoms • Diamonds are made of atoms of the element Carbon • Foil is made of the atoms of the element Aluminum.

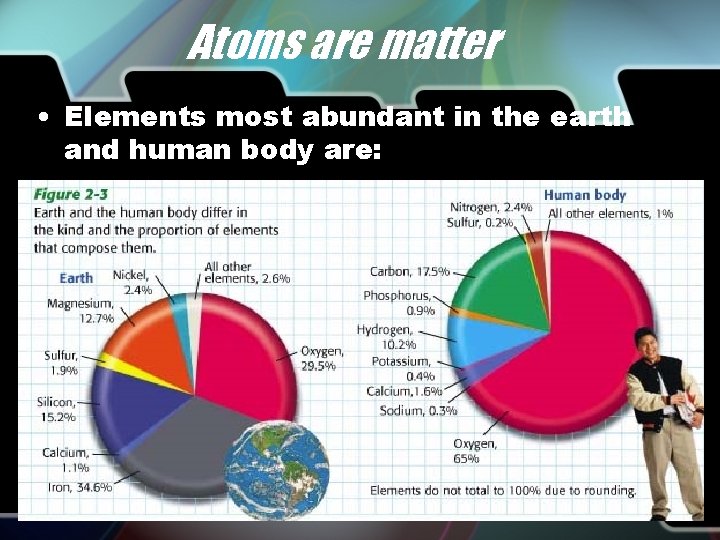
Atoms are matter • Elements most abundant in the earth and human body are:

Elements • Pure substance that consists entirely of one type of atom Hydrogen Sulfur Magnesium

COMPOUNDS • 2 or more elements combine to make a compound • A compound is a substance that can be separated into simpler substances only by chemical means

COMPOUNDS • Examples: Water - H 20 Salt - Na. Cl Hydrogen Peroxide - H 2 O 2

Molecules • Smallest unit of a substance that exhibits all of the properties characteristic of that substance • They act as a unit – Example: water H 2 O

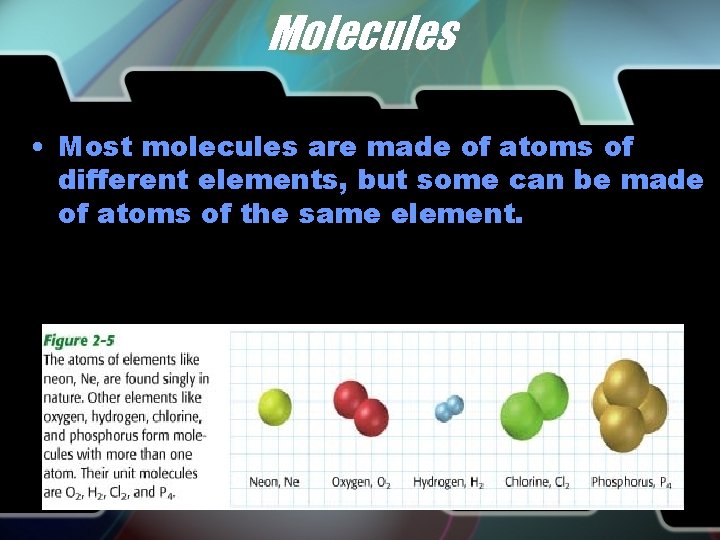
Molecules • Most molecules are made of atoms of different elements, but some can be made of atoms of the same element.

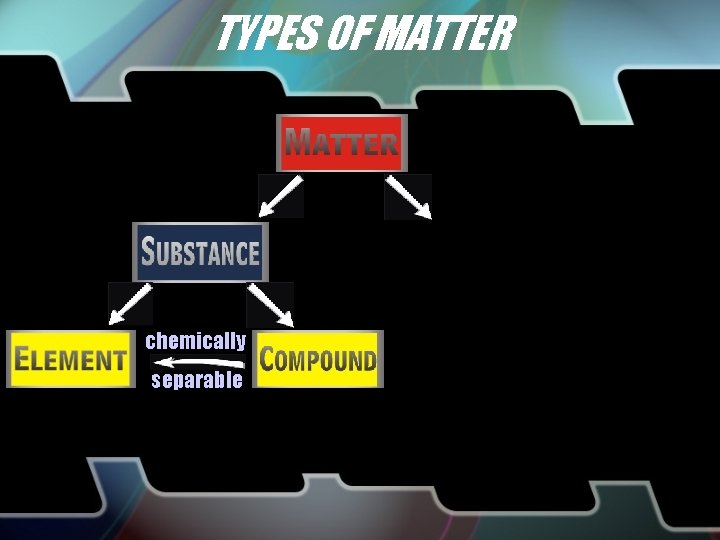
TYPES OF MATTER chemically separable

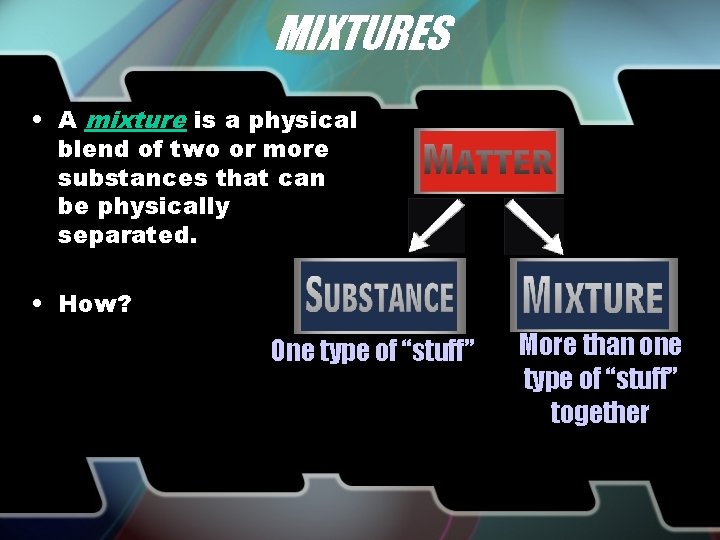
MIXTURES • A mixture is a physical blend of two or more substances that can be physically separated. • How? One type of “stuff” More than one type of “stuff” together

Pure Substance vs. Mixture • When people say “Pure Grape Juice” this means it contains only the juice of the grapes with NOTHING added or taken away • In reality, grape juice is NOT a pure substance. It is a MIXTURE!!!

Mixture • A combination of more than one substance – Grape juice is a mixture because it contains water, sugars, acids, and vitamins – The composition of grape juice is not fixed; it can have different amounts of water, sugars, or other compounds

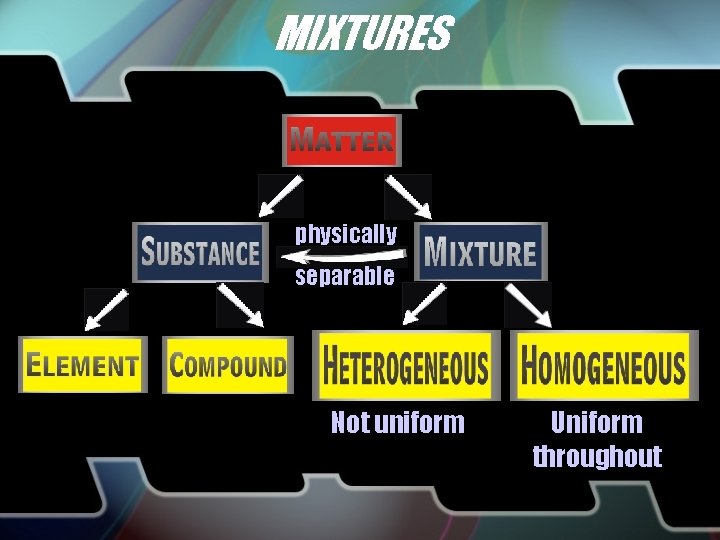
MIXTURES physically separable Not uniform Uniform throughout

HETEROGENEOUS vs. HOMOGENEOUS • There are 2 different types of mixtures. – A heterogeneous mixture has more than one phase or substance (looks different) – A homogeneous mixture has only one phase (looks the same) • Also called a SOLUTION

Heterogeneous Mixtures • Mixtures of this type are not the same throughout. – Example: Fruit Salad • Each spoonful of fruit salad would give you a different variety – no 2 spoonfuls would be exactly the same.

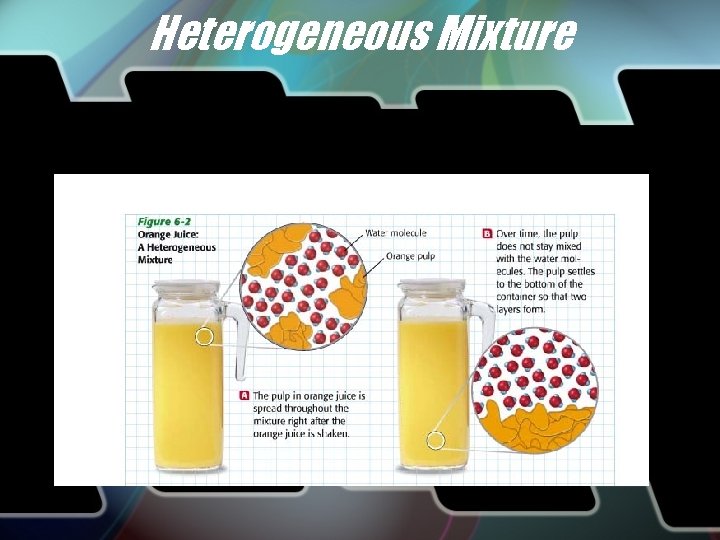
Heterogeneous Mixture

SUSPENSION a mixture that looks uniform when stirred or shaken that separates into different layers when it is no longer agitated

COLLOID • Gelatin heterogeneous mix – Gelatin is a colloid – Colloid a mixture of very tiny particles of pure substances that are dispersed in another substance but do not settle out of the substance

Colloids • Differences between suspensions and colloids: • Particles in colloids are much smaller • Because they are so small, they do NOT separate out or settle to the bottom in colloids • Particles stay dispersed throughout the mixture

Colloids • EXAMPLES: • • • Fog Milk Shaving cream Egg white Paint Blood

Types of Heterogeneous Mixtures • Colloids – Particles never settle – Displays the Tyndall Effect • Scattering of light particles • Ex) Fog, milk, shaving cream

Homogeneous Mixtures • These mixtures are uniform, in that no matter where you get your sample from in the mixture – each sample will be the exact same. – Examples: Kool-Aid, tomato soup, salt water, brass keys

Homogeneous Mixtures • Not only look uniform, they ARE uniform • Ex: Salt water • If you add salt to a glass of pure water and mix it, it will eventually look like pure water • Looks uniform because the components of the mixture are too small to be seen

Homogeneous Mixtures • When salt and water mix, no chemical reaction takes place • Easy to separate the 2 substances by evaporating or boiling the water • Once boiled, only left with salt

homogeneous heterogeneous

Solutions and Homogeneous mixtures SOLUTIONS & HOMOGENEOUS MIXTURES are synonymous. Homogenous mixtures are mixed completely, all the way down to their most fundamental particles-atoms, molecules, or ions.

Types of Heterogeneous Mixtures • Colloids – Particles never settle – Displays the Tyndall Effect • Scattering of light particles • Ex) Fog, milk, shaving cream • Suspensions – Visible particles settle – Require agitation to stay mixed • Ex) OJ, Italian dressing, sand & water

HETEROGENEOUS OR HOMOGENEOUS? • Saltwater Homogeneous • Spaghetti sauce • Muddy water Heterogeneous • Cough syrup Homogeneous • Salad • Brass Heterogeneous Homogeneous