Components of Organic Chemistry Reactions synthesis Organic compounds
![v Deprotonation Equilibrium K K = [A: ][BH] / [HA][B: ] = [A: ]/[HA] v Deprotonation Equilibrium K K = [A: ][BH] / [HA][B: ] = [A: ]/[HA]](https://slidetodoc.com/presentation_image_h2/023c9a04b6f5fa2093c2dc56315cfa0e/image-4.jpg)


- Slides: 26

Components of Organic Chemistry Reactions: synthesis Organic compounds Structure bonding, conformation, analysis, stereochem. Carey B-5 ed-Chap 1 Reactivity interaction with other molecules: mechanism, dynamic stereochem. 1

Chapter 1. Alkylation: What to study r Generation of carbon nucleophiles uenolate r & imine anions: conditions of formation Alkylation: reaction conditions ureactivity r and structure of carbon nucleophile Selectivity of alkylation uregioselectivity Carey B-5 ed-Chap 1 & stereoselectivity 2

Generation & Properties of Enolates r Generation of enolates: acid-base reactions udeprotonation: uacidity stronger bases for enough enolates of C-H (p. Ka): inductive & resonance; 3 Table 1. 1 m the more acidic, the faster deprotonation (kinetic) & the more stable carbanion (thermodynamic) ubases: HOK, RONa & LDA, L(Na/K)HMDS, Na(K)H, RLi usolvents: Carey B-5 ed-Chap 1 ROH & ether, THF, DME, DMF, DMSO, Ph. Me 3
![v Deprotonation Equilibrium K K A BH HAB A HA v Deprotonation Equilibrium K K = [A: ][BH] / [HA][B: ] = [A: ]/[HA]](https://slidetodoc.com/presentation_image_h2/023c9a04b6f5fa2093c2dc56315cfa0e/image-4.jpg)
v Deprotonation Equilibrium K K = [A: ][BH] / [HA][B: ] = [A: ]/[HA] x [BH]/[B: ] = [A: ][H 3 O+]/[HA] x [BH]/[B: ][H 3 O+] = Ka(HA) / Ka(HB) = 10 exp(-p. Ka(HA) + p. Ka(HB) ) Ka (HA) = [A: ][H 3 O+] / [HA] Ka (HB) = [B: ][H 3 O+] / [HB] K > 1, when HA is stronger than HB (p. Ka (HA) < p. Ka (HB)) m DG Carey B-5 ed-Chap 1 = - RT ln K < 0; K= 10(-20 + 35) = 1015 4

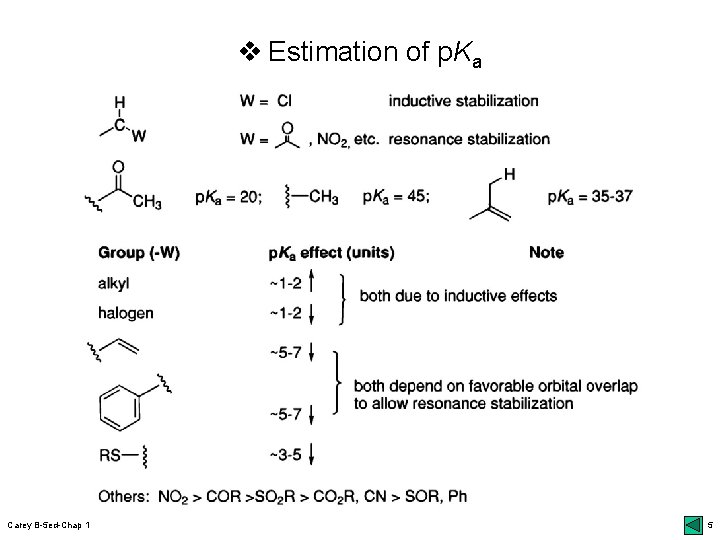
v Estimation of p. Ka Carey B-5 ed-Chap 1 5

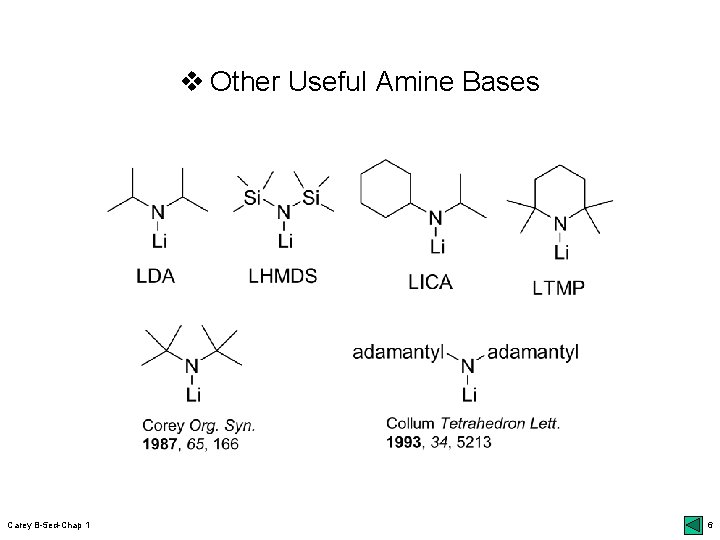
v Other Useful Amine Bases Carey B-5 ed-Chap 1 6

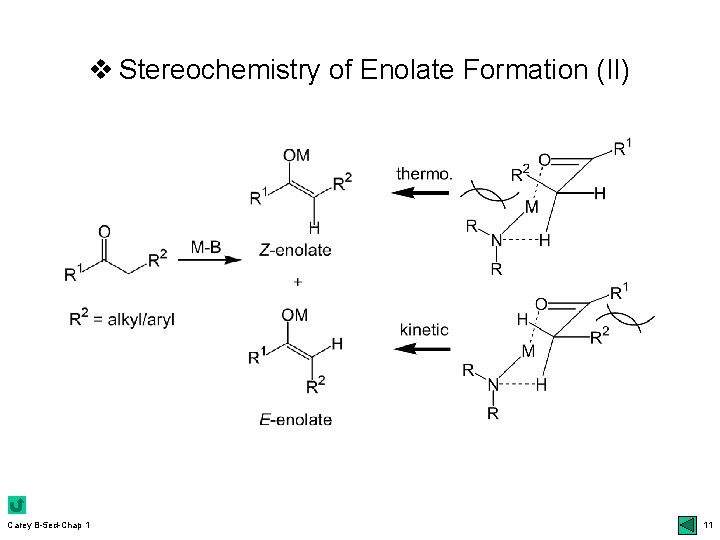
v Regioselectivity in Enolate Formation r Kinetic vs thermodynamic control: 6 top & others ukinetic control: rapid, irreversible, quantitative deprotonation m more acidic H at the less substituted carbon (bases) m aprotic solvent, excess amount of strong bases, more covalent Li-O than Na/K, low temp. & short rxn time uthermodynamic m more utypical substituted & conjugated enolates examples: 7 -8 Scheme 1. 1 uconjugated Carey B-5 ed-Chap 1 control: more stable enolates (equilibration) ketones: kinetic (a-C) & thermo (g-C); 13 top 7

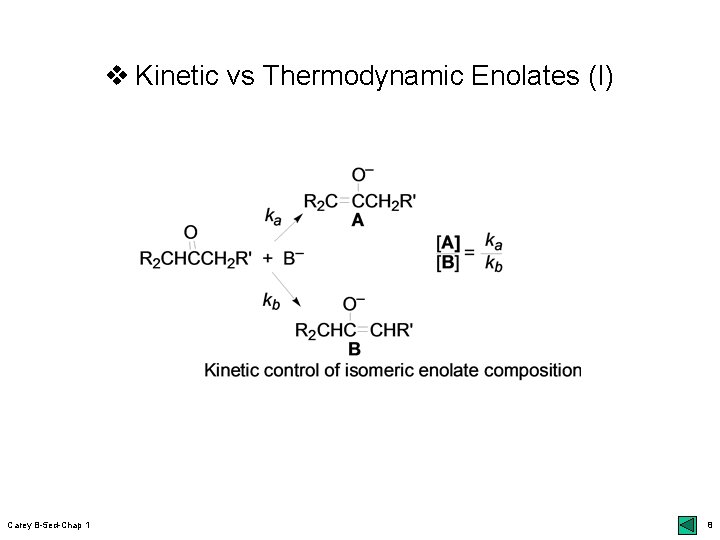
v Kinetic vs Thermodynamic Enolates (I) Carey B-5 ed-Chap 1 8

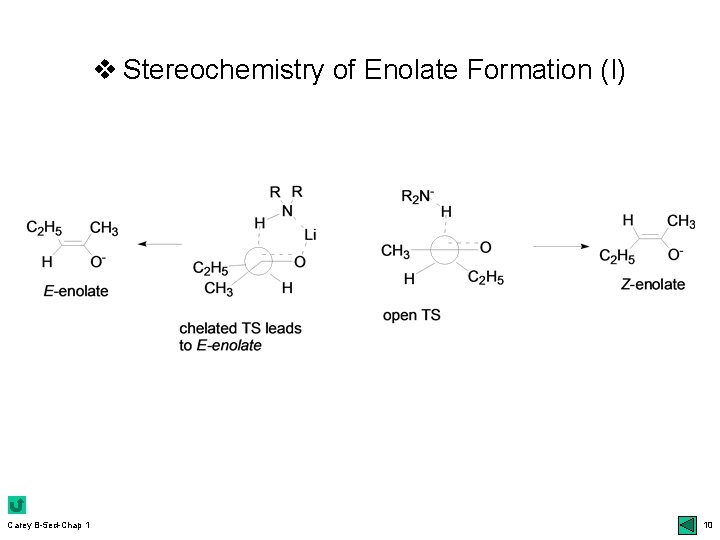
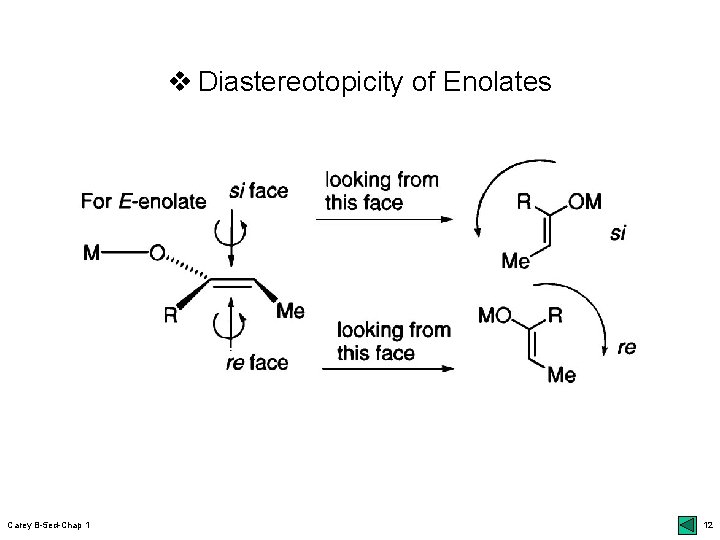
v Stereoselectivity in Enolate Formation r E- vs Z-enolates: closed vs open TS; 9 bottom udefinition of E & Z: higher priority for the oxygen bonded to metal regardless of the normal priority of the other atom uclosed TS: kinetic control; Ireland models udiastereoselectivity: m chelation 10 top; 12 Table 1. 2 effect of LHMDS (Z) vs steric effect (E): 12 bot r Diastereotopic faces of enolates: si vs re face r Enantioselective deprotonation: 13 bottom ukinetic umore Carey B-5 ed-Chap 1 resolution: mechanism; 14 top chiral bases: 13 middle 9

v Stereochemistry of Enolate Formation (I) Carey B-5 ed-Chap 1 10

v Stereochemistry of Enolate Formation (II) Carey B-5 ed-Chap 1 11

v Diastereotopicity of Enolates Carey B-5 ed-Chap 1 12

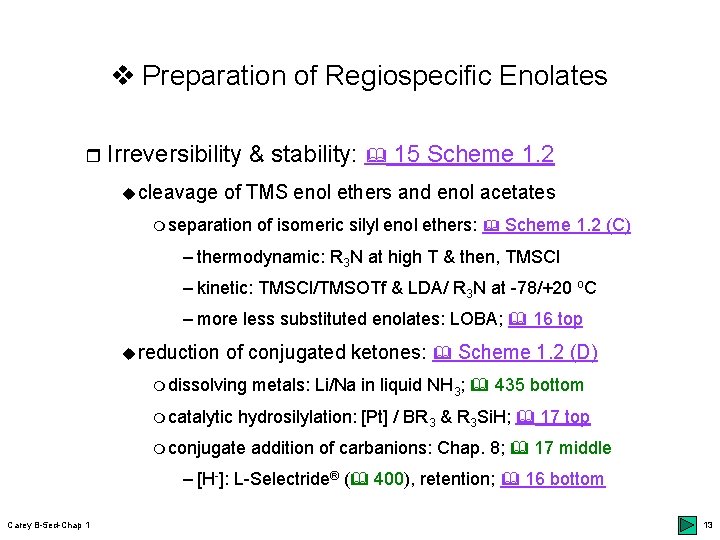
v Preparation of Regiospecific Enolates r Irreversibility & stability: 15 Scheme 1. 2 ucleavage of TMS enol ethers and enol acetates m separation of isomeric silyl enol ethers: Scheme 1. 2 (C) – thermodynamic: R 3 N at high T & then, TMSCl – kinetic: TMSCl/TMSOTf & LDA/ R 3 N at -78/+20 o. C – more less substituted enolates: LOBA; 16 top ureduction of conjugated ketones: Scheme 1. 2 (D) m dissolving m catalytic metals: Li/Na in liquid NH 3; 435 bottom hydrosilylation: [Pt] / BR 3 & R 3 Si. H; 17 top m conjugate addition of carbanions: Chap. 8; 17 middle – [H-]: L-Selectride® ( 400), retention; 16 bottom Carey B-5 ed-Chap 1 13

v Aggregation Factors in Alkylation of Enolates r Solvent effects: 17 Table 1. 3 ustructure uhighly of enolates: aggregates; 19 Fig. 1. 2 -1. 3 polar aprotic solvents: ‘naked (bare) ions’; 18 m strong solvation to a metal cation & weak to an enolate ion uless polar aprotic solvents: lower reactivity but more useful (workup & kinetic enolates); THF, ether, DME m enhanced reactivity with chelating additives: TMEDA, HMPA, crown ethers (18 -crown-6: K, Na; 12 -crown-4: Li); 21 top – enhanced alkylation rate: 20 bottom r Counter cation: reactivity; K+ > Na+ > Li+ > Mg 2+ usmaller, Carey B-5 ed-Chap 1 harder cations: tightly associated with enolates 14

v Indirect Alkylation of Enolates r Enolates with two M groups: 22 Scheme 1. 3 ugood (reliable) Nu produced by weak bases: RO- agents: a-halocarbonyl > benzyl/allyl > 1 o halides (sulfonates) > 2 o halides, no 3 o halides m alkylating m dialkylation: m cyclization one-pot or stepwise (two-step); side rxns with dihaloalkanes (entry 6): 3 -ring > 5 > 6 > 4 udecarboxylation: synthetic equivalents to enolates with one M group; 23 top & 23 Scheme 1. 4 m dianion of acetoacetic acid: no hydrolysis; 24 top m dianions Carey B-5 ed-Chap 1 of monoalkyl malonates: 24 middle 15

v Direct Alkylation of Enolates: Ketones r No decarboxylation required: 29 Scheme 1. 7 uquantitative & selective formation of enolates: strong & soluble bases, controlled reaction conditions r Stereoselectivity in alkylation of enolates: 29 -30 ustereoelectronic m axial substitution more favored with R at C-2: 25 middle m endocyclic m exocyclic enolates: steric; trans to R on the ring enolates: steric or stereoelectronic (axial) ubicyclic examples: 26 middle - 27 top uacyclic enolates: allylic strain (A 1, 3); 28 middle uconjugated Carey B-5 ed-Chap 1 control: orbital overlap in space enolates: kinetic a-alkylation; 31 top 16

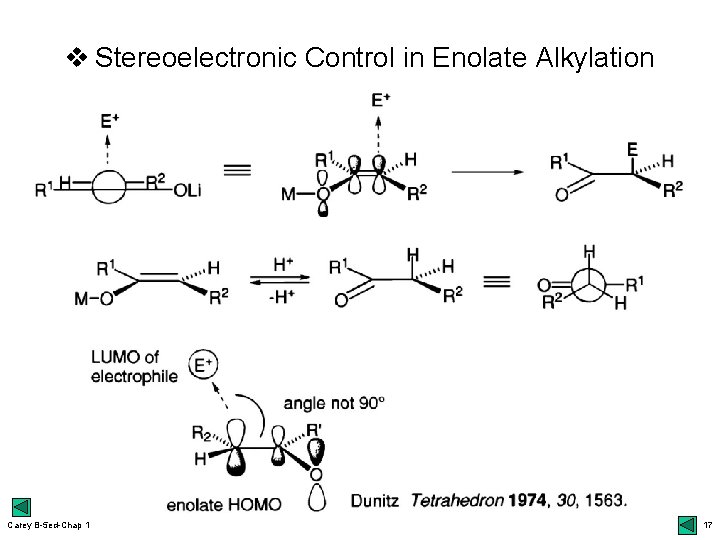
v Stereoelectronic Control in Enolate Alkylation Carey B-5 ed-Chap 1 17

v Alkylation of Other Carbonyls: 34 -35 r Esters & amides: high p. Ka & condensation (Claisen) ustrong r amide bases at low temp & addition of HMPA unitriles: less self-condensation products umalate enolates: chelation control; 33 middle & Fig. 1. 5 Aldehydes: undesired aldol condensations urapid & quantitative formation of enolates: 31 bottom m better r alkylation via enamines or imine anions: Chap. 1. 3 carboxylic acids: via dianions; 34 top u 1, 3 -dicarbonyls: Carey B-5 ed-Chap 1 different reactivities; 37 Scheme 1. 7 18

v Intramolecular Alkylation of Enolates r Ring size & geometric requirements: 37 -38 ucloseness of electrophilic sites to Nu: 3 > 5 > 6 > 7 > 4 u. Baldwin’s rule: ease of ring formation; 5 -ring vs 6 -ring m DG‡ = DH‡ (strain factor) - TDS‡ (distance factor); exam!! m trajectory uester Carey B-5 ed-Chap 1 of Nu & hybridization of the electrophilic carbon enolates: H-eclipsed & R-pseudoequatorial; 38 -40 19

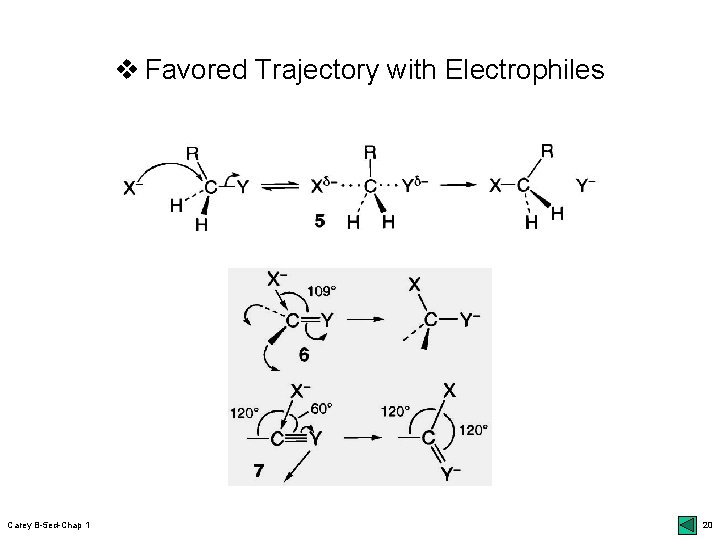
v Favored Trajectory with Electrophiles Carey B-5 ed-Chap 1 20

v Enantioselective Alkylation of Enolates r Facial control with chiral auxiliaries: 41 u. Evans’ oxazolidinones: Z-enolates; 41 middle uacyclic amino alcohols: chelation control; 42 uremote control: p-p interaction; 43 top & Scheme 1. 9 ubicyclic enolates: bicyclic lactams; 45 top & middle uother Carey B-5 ed-Chap 1 chiral auxiliaries: substrate control & reagent control 21

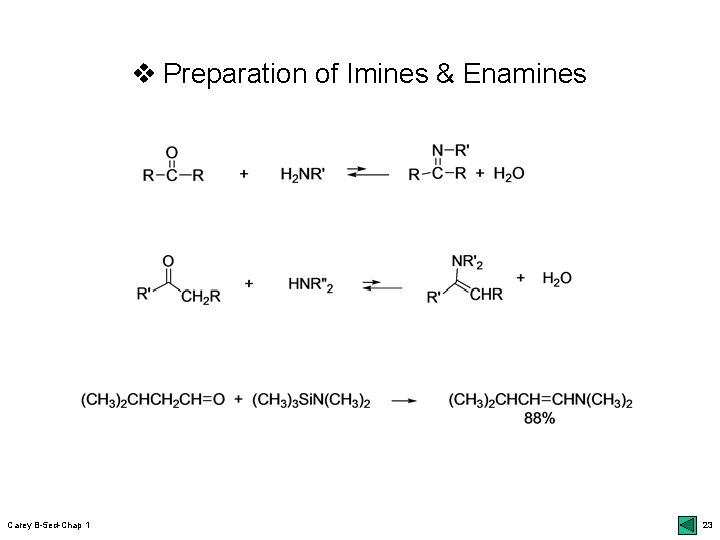
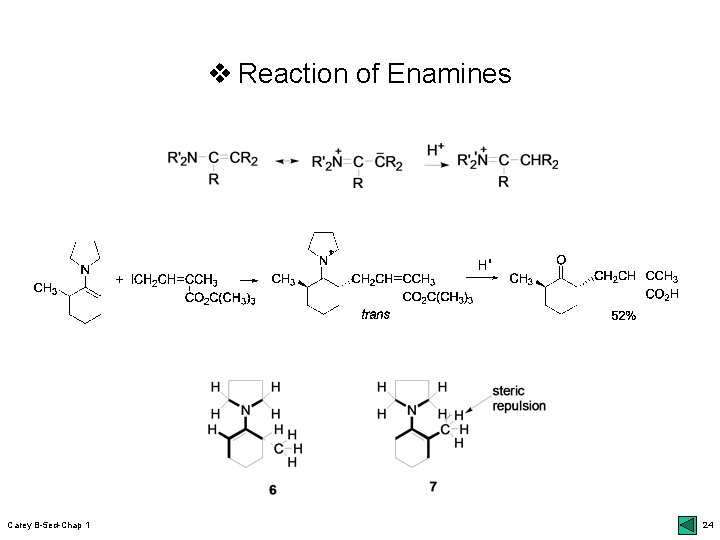
v Nitrogen Analogs of Enols and Enolates r Imines: azomethines or Schiff bases; 46 top upreparation: r 1 o amines & ketones/aldehydes; dehydration Enamines (vinylamines): 2 o amines; 46 middle ufacile dehydration: Ti. Cl 4 or R 3 Si-NR’ 2; oxophilic atoms umore basic than enols: reactive E+; 46 bot & Scheme 1. 10 uregio-/stereoselective r alkylation: less & trans; 47 bottom Imine anions: metalloenamine; 49 middle ustructure of azaallyl anions: ionic N atom; 49 Fig. 1. 6 umore reactive than enolates & less condensation with aldehydes: 50 top & side reactions of enolates Carey B-5 ed-Chap 1 22

v Preparation of Imines & Enamines Carey B-5 ed-Chap 1 23

v Reaction of Enamines Carey B-5 ed-Chap 1 24

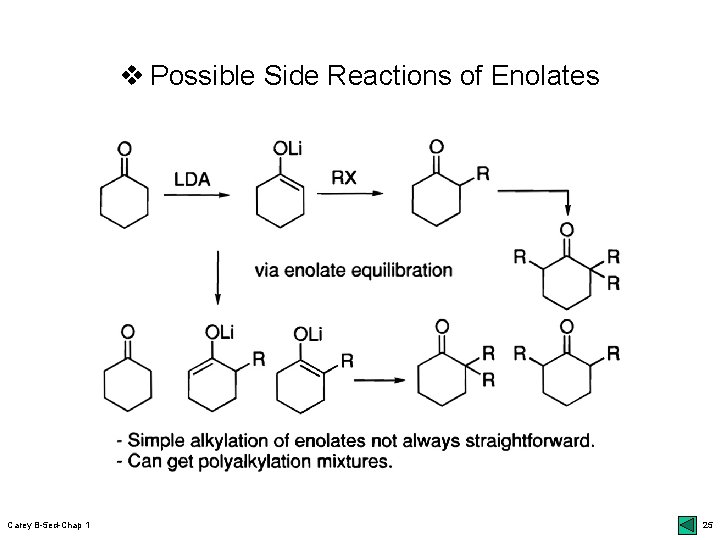
v Possible Side Reactions of Enolates Carey B-5 ed-Chap 1 25
