Complex Trial Designs Ada Keding Statistician York Trials










- Slides: 39

Complex Trial Designs Ada Keding Statistician – York Trials Unit BOA Orthopaedic Surgery Research Centre

Overview • Two Design Approaches (Explanatory vs Pragmatic) • Considerations for going beyond the Standard Trial Design • Complex Trial Designs – Examples and Evaluation

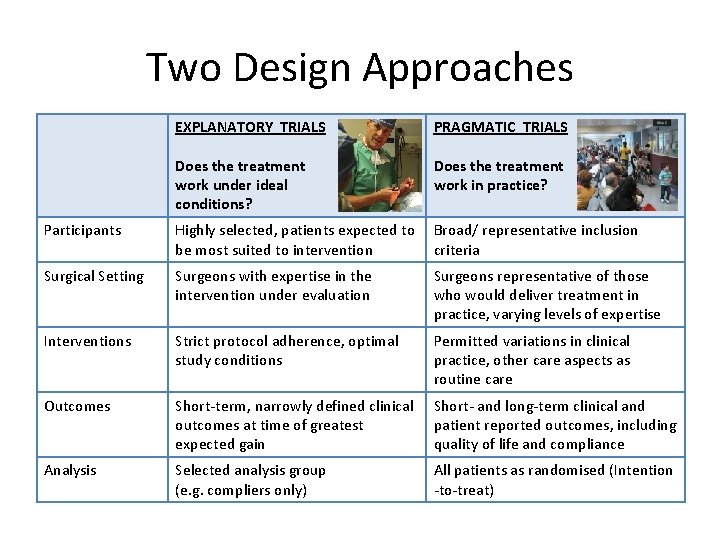
Two Design Approaches EXPLANATORY TRIALS PRAGMATIC TRIALS Does the treatment work under ideal conditions? Does the treatment work in practice? Participants Highly selected, patients expected to be most suited to intervention Broad/ representative inclusion criteria Surgical Setting Surgeons with expertise in the intervention under evaluation Surgeons representative of those who would deliver treatment in practice, varying levels of expertise Interventions Strict protocol adherence, optimal study conditions Permitted variations in clinical practice, other care aspects as routine care Outcomes Short-term, narrowly defined clinical outcomes at time of greatest expected gain Short- and long-term clinical and patient reported outcomes, including quality of life and compliance Analysis Selected analysis group (e. g. compliers only) All patients as randomised (Intention -to-treat)

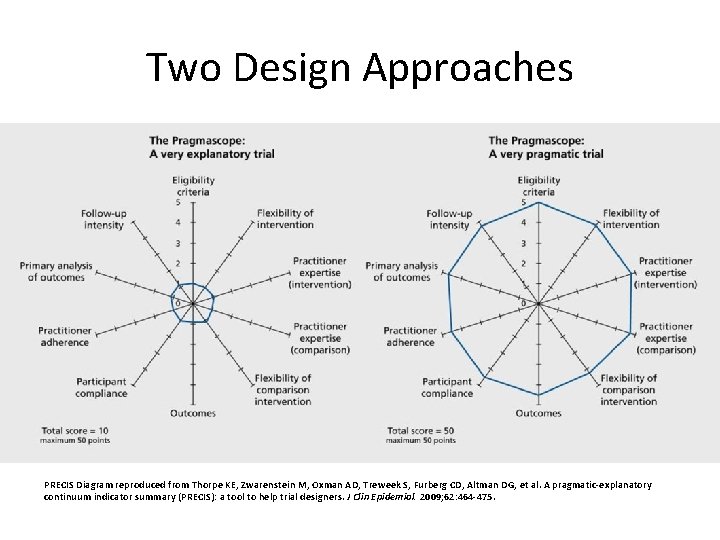
Two Design Approaches PRECIS Diagram reproduced from Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol. 2009; 62: 464 -475.

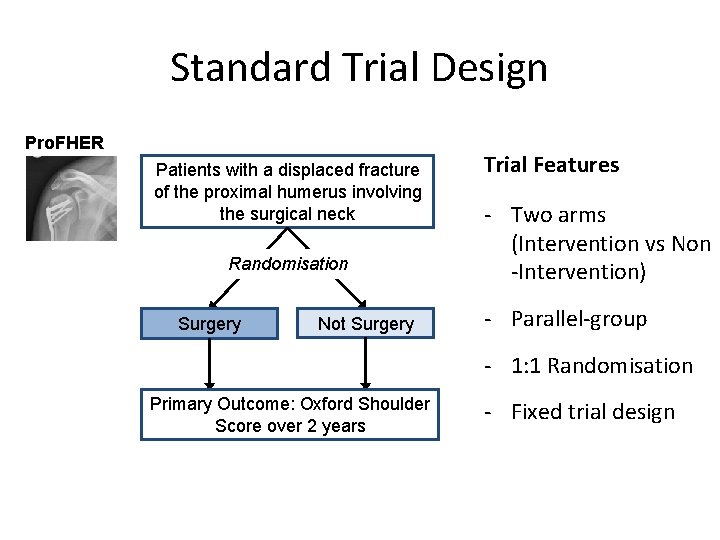
Standard Trial Design Pro. FHER Patients with a displaced fracture of the proximal humerus involving the surgical neck Randomisation Surgery Not Surgery Trial Features - Two arms (Intervention vs Non -Intervention) - Parallel-group - 1: 1 Randomisation Primary Outcome: Oxford Shoulder Score over 2 years - Fixed trial design

Design Considerations • Number of treatments of interest • Size of available patient population; likely consent and dropout rates • Logistics of randomisation and treatment implementation, including available resources (equipment / expertise) • Prior attitudes and beliefs of patients and clinicians • Expected sources of bias • Urgency of scientific evidence

Complex Trial Designs - Overview • Logistic Considerations – Cluster – Crossover • Testing Multiple Interventions – Multi-Arm – Factorial – Adaptive • Taking Individual Preferences into Account – Patient Preference – Expertise Based

Cluster Trials • Sometimes not practical to implement randomisation at the patient level (e. g. logistics for specialised equipment or staff level interventions) • Certain interventions may be susceptible to contamination • Possible solution: Randomisation at a higher level (e. g. the ward or hospital) • The unit of analysis remains the patient, with the clustering around the randomised groupings accounted for statistically

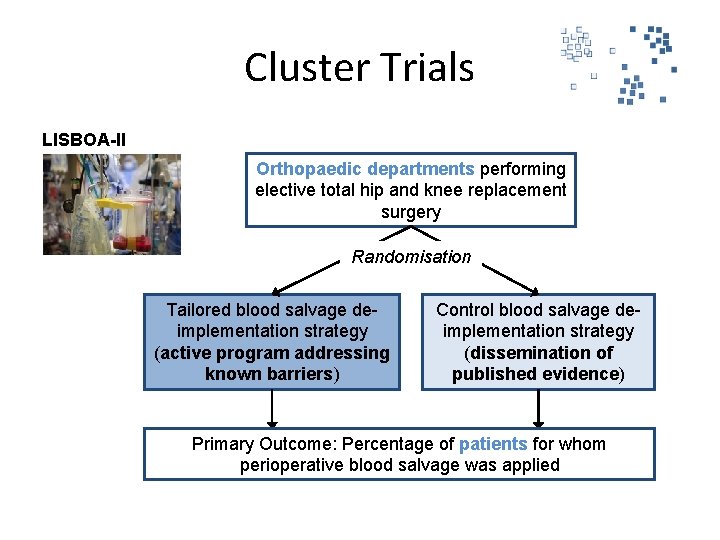
Cluster Trials LISBOA-II Orthopaedic departments performing elective total hip and knee replacement surgery Randomisation Tailored blood salvage deimplementation strategy (active program addressing known barriers) Control blood salvage deimplementation strategy (dissemination of published evidence) Primary Outcome: Percentage of patients for whom perioperative blood salvage was applied

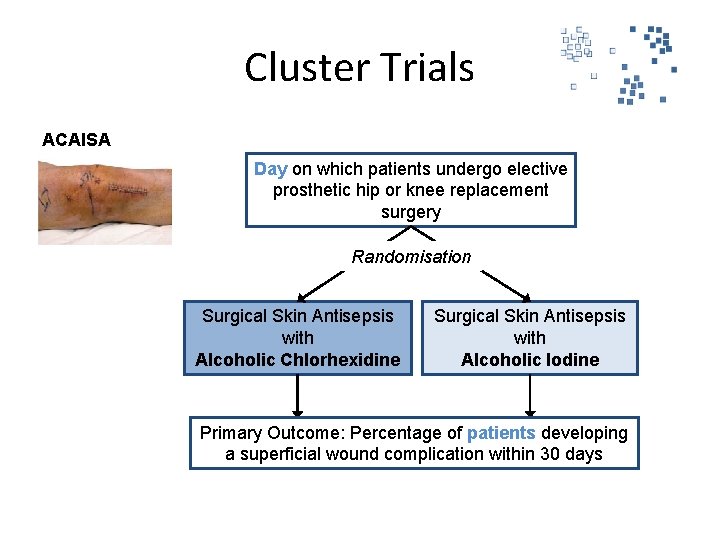
Cluster Trials ACAISA Day on which patients undergo elective prosthetic hip or knee replacement surgery Randomisation Surgical Skin Antisepsis with Alcoholic Chlorhexidine Surgical Skin Antisepsis with Alcoholic Iodine Primary Outcome: Percentage of patients developing a superficial wound complication within 30 days

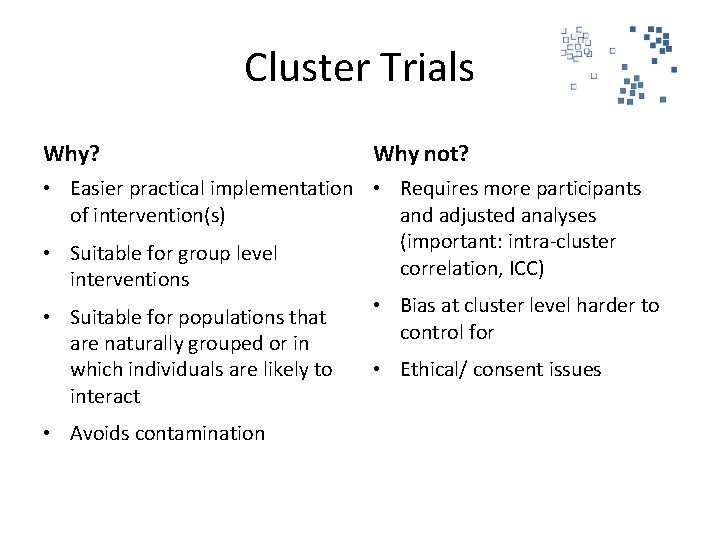
Cluster Trials Why? Why not? • Easier practical implementation • Requires more participants of intervention(s) and adjusted analyses (important: intra-cluster • Suitable for group level correlation, ICC) interventions • Bias at cluster level harder to • Suitable for populations that control for are naturally grouped or in which individuals are likely to • Ethical/ consent issues interact • Avoids contamination

Crossover Trials • Depending on the type of intervention, it may be advantageous for patients to receive both trial treatments, especially if only a small number of participants is available • In crossover trials, the order in which each treatment is received is randomised, rather than the treatments themselves

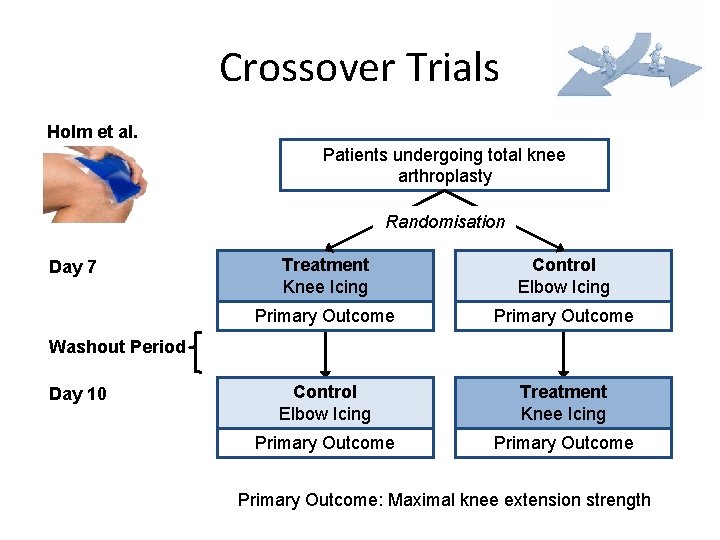
Crossover Trials Holm et al. Patients undergoing total knee arthroplasty Randomisation Day 7 Treatment Knee Icing Control Elbow Icing Primary Outcome Control Elbow Icing Treatment Knee Icing Primary Outcome Washout Period Day 10 Primary Outcome: Maximal knee extension strength

Crossover Trials Why? Why not? • Each participant acts as their own control • Period effects • More precise treatment effect • Carry-over effects • Fewer participants needed • Not suitable for curative treatments • Patients get to try both treatments • Treatment related retention issues • Applicable to chronic conditions • Patients required for longer

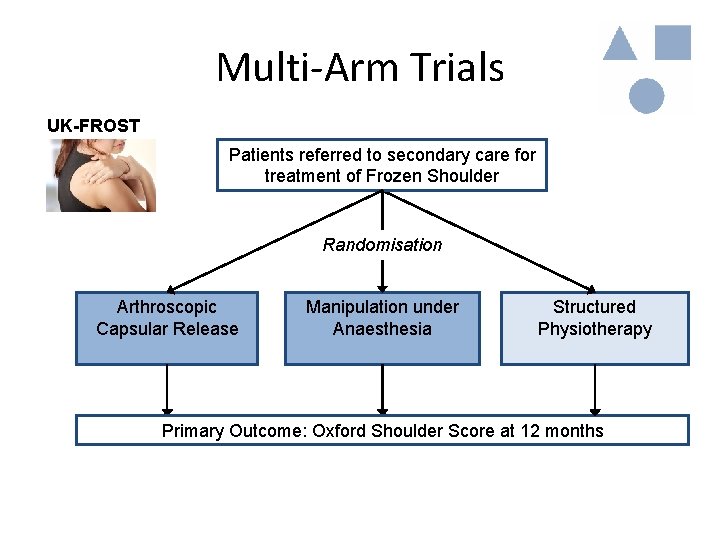
Multi-Arm Trials • Often there a number of alternative treatments of interest – Are treatments A, B, C superior to the Control treatment? – Are treatments A, B, C equally effective? • Possible solution: Randomise patients to multiple treatment arms

Multi-Arm Trials UK-FROST Patients referred to secondary care for treatment of Frozen Shoulder Randomisation Arthroscopic Capsular Release Manipulation under Anaesthesia Structured Physiotherapy Primary Outcome: Oxford Shoulder Score at 12 months

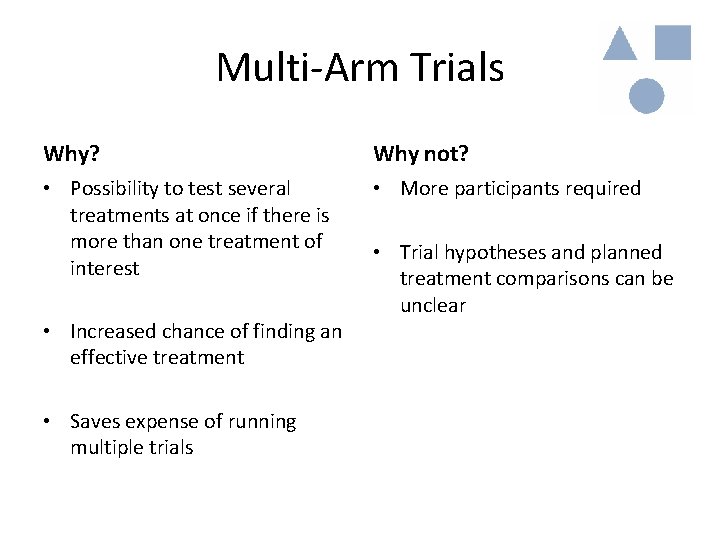
Multi-Arm Trials Why? Why not? • Possibility to test several treatments at once if there is more than one treatment of interest • More participants required • Increased chance of finding an effective treatment • Saves expense of running multiple trials • Trial hypotheses and planned treatment comparisons can be unclear

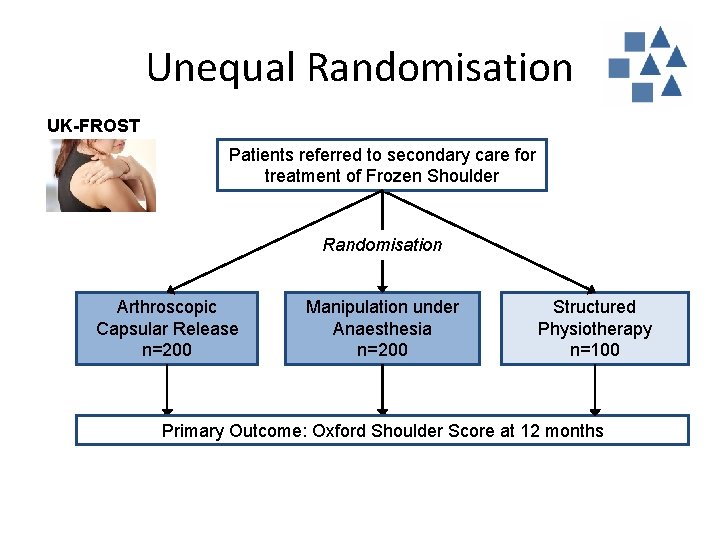
Unequal Randomisation UK-FROST Patients referred to secondary care for treatment of Frozen Shoulder Randomisation Arthroscopic Capsular Release n=200 Manipulation under Anaesthesia n=200 Structured Physiotherapy n=100 Primary Outcome: Oxford Shoulder Score at 12 months

Unequal Randomisation Why? Why not? • Maximum exposure to / evidence gathering for experimental interventions • Unequal numbers and variability in data may affect power and precision of analysis, but usually outweighed by applicable benefit • Allow for learning curve of new techniques • Minimise costs for expensive treatment options • Differential sought effect sizes between multiple treatment arms • Other practical reasons • Unless justified by specific trial circumstances, equal allocation is desirable

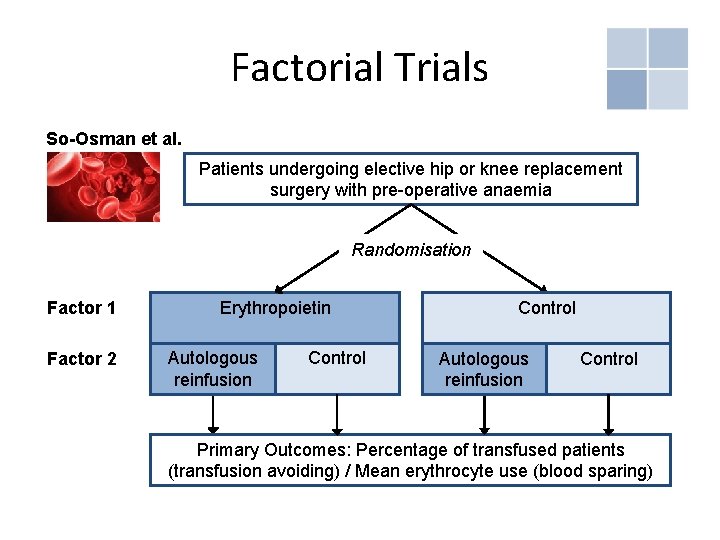
Factorial Trials • Not always feasible to have large number of patients available to investigate multiple treatment arms, also does not allow investigation of combined effects • Possible solution: Randomise patients to receive one, none or combination of treatments • Referred to as n x n factorial designs (e. g. 2 x 2 = two investigations/factors with two treatment arms each)

Factorial Trials So-Osman et al. Patients undergoing elective hip or knee replacement surgery with pre-operative anaemia Randomisation Factor 1 Factor 2 Erythropoietin Autologous reinfusion Control Primary Outcomes: Percentage of transfused patients (transfusion avoiding) / Mean erythrocyte use (blood sparing)

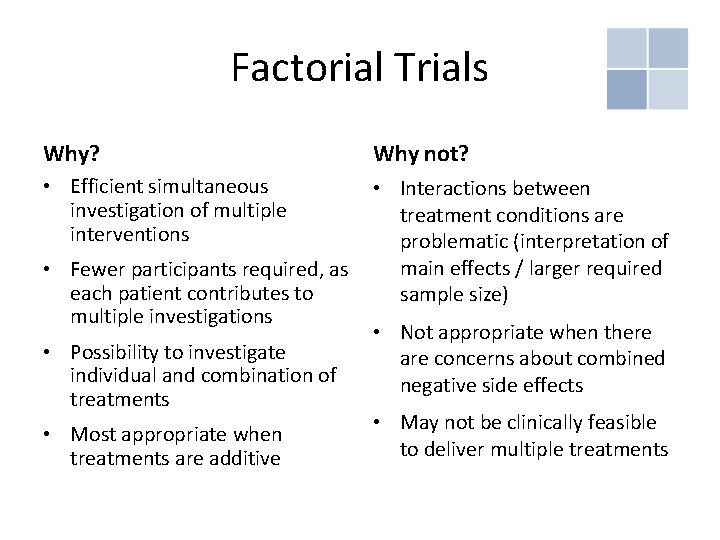
Factorial Trials Why? Why not? • Efficient simultaneous investigation of multiple interventions • Interactions between treatment conditions are problematic (interpretation of main effects / larger required sample size) • Fewer participants required, as each patient contributes to multiple investigations • Possibility to investigate individual and combination of treatments • Most appropriate when treatments are additive • Not appropriate when there are concerns about combined negative side effects • May not be clinically feasible to deliver multiple treatments

Adaptive Designs • Advances in medical knowledge can be slow with traditional trial designs – Many interventions to evaluate, large proportion of which are likely to be ineffective – Many aspects of treatments to test – Phase III trials require enormous time and effort • Possible solution: more flexible ‘adaptive’ designs – Many different types – More prominent in industry drug trials, but increasingly popular for other medical interventions

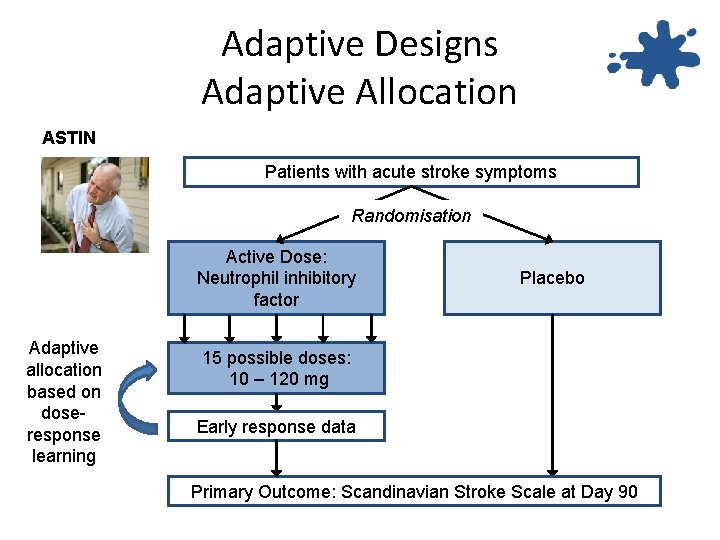
Adaptive Designs Adaptive Allocation ASTIN Patients with acute stroke symptoms Randomisation Active Dose: Neutrophil inhibitory factor Adaptive allocation based on doseresponse learning Placebo 15 possible doses: 10 – 120 mg Early response data Primary Outcome: Scandinavian Stroke Scale at Day 90

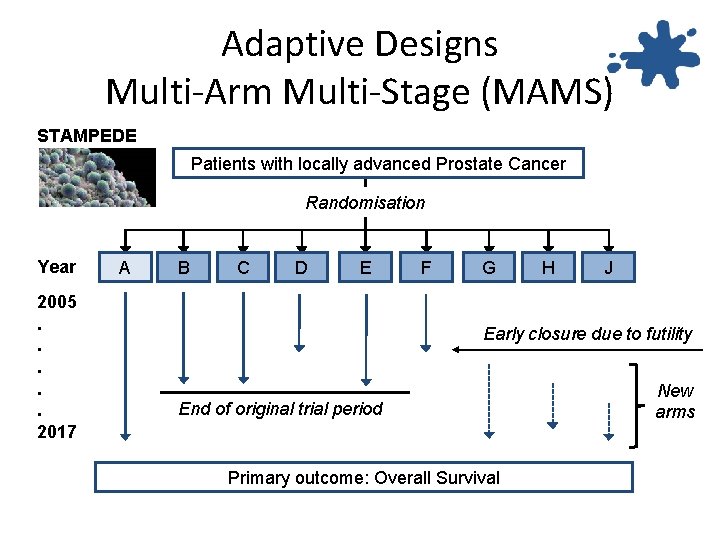
Adaptive Designs Multi-Arm Multi-Stage (MAMS) STAMPEDE Patients with locally advanced Prostate Cancer Randomisation Year 2005. . . 2017 A B C D E F G H J Early closure due to futility End of original trial period Primary outcome: Overall Survival New arms

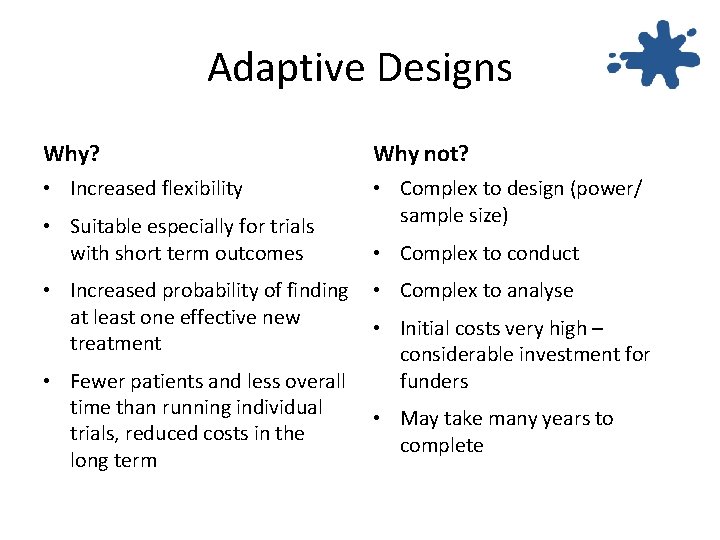
Adaptive Designs Why? Why not? • Increased flexibility • Complex to design (power/ sample size) • Suitable especially for trials with short term outcomes • Increased probability of finding at least one effective new treatment • Fewer patients and less overall time than running individual trials, reduced costs in the long term • Complex to conduct • Complex to analyse • Initial costs very high – considerable investment for funders • May take many years to complete

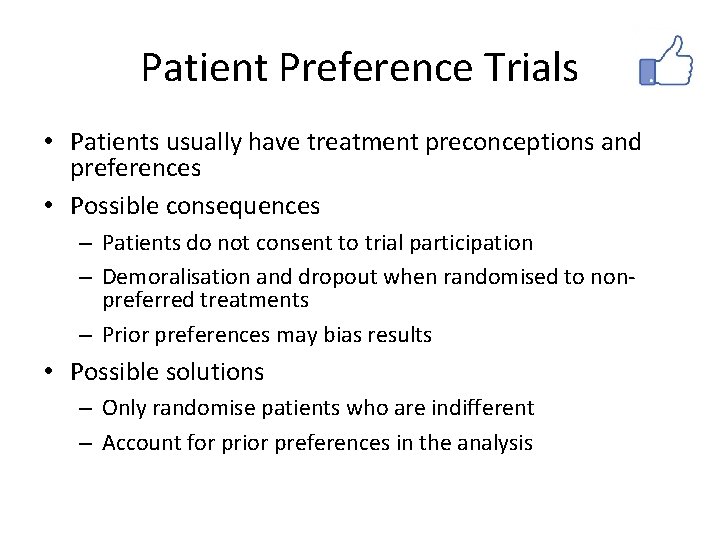
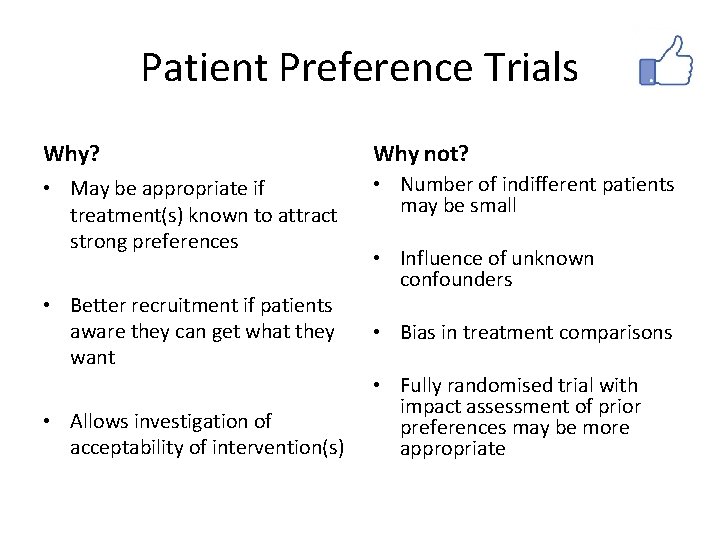
Patient Preference Trials • Patients usually have treatment preconceptions and preferences • Possible consequences – Patients do not consent to trial participation – Demoralisation and dropout when randomised to nonpreferred treatments – Prior preferences may bias results • Possible solutions – Only randomise patients who are indifferent – Account for prior preferences in the analysis

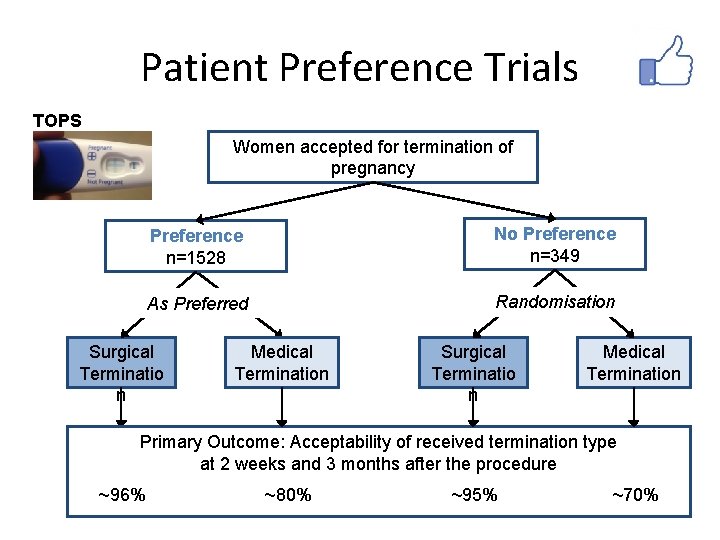
Patient Preference Trials TOPS Women accepted for termination of pregnancy Preference n=1528 No Preference n=349 As Preferred Randomisation Surgical Terminatio n Medical Termination Primary Outcome: Acceptability of received termination type at 2 weeks and 3 months after the procedure ~96% ~80% ~95% ~70%

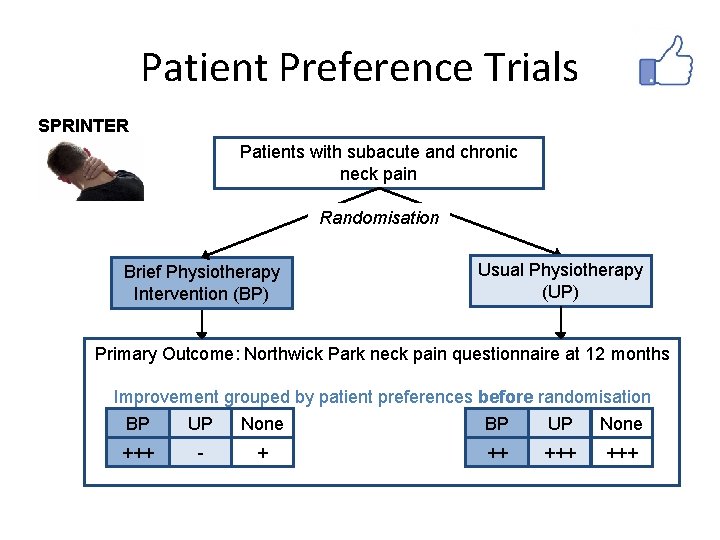
Patient Preference Trials SPRINTER Patients with subacute and chronic neck pain Randomisation Brief Physiotherapy Intervention (BP) Usual Physiotherapy (UP) Primary Outcome: Northwick Park neck pain questionnaire at 12 months Improvement grouped by patient preferences before randomisation BP UP None +++ - + ++ +++

Patient Preference Trials Why? Why not? • May be appropriate if treatment(s) known to attract strong preferences • Number of indifferent patients may be small • Better recruitment if patients aware they can get what they want • Allows investigation of acceptability of intervention(s) • Influence of unknown confounders • Bias in treatment comparisons • Fully randomised trial with impact assessment of prior preferences may be more appropriate

Expertise Based Trials • Surgeons are unlikely to have equivalent expertise in a wide variety of techniques • Based on personal experience and expertise, some surgeons may not be in equipoise • Conventional randomisation may lead to – Surgeries being performed by less experienced or less motivated clinicians – Differential expertise bias • Possible solution: Randomise patients to a surgeon with defined expertise level of a trial treatment

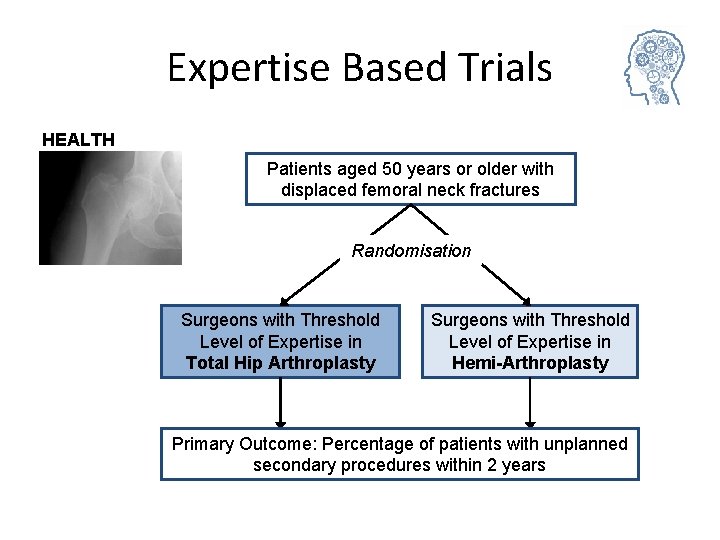
Expertise Based Trials HEALTH Patients aged 50 years or older with displaced femoral neck fractures Randomisation Surgeons with Threshold Level of Expertise in Total Hip Arthroplasty Surgeons with Threshold Level of Expertise in Hemi-Arthroplasty Primary Outcome: Percentage of patients with unplanned secondary procedures within 2 years

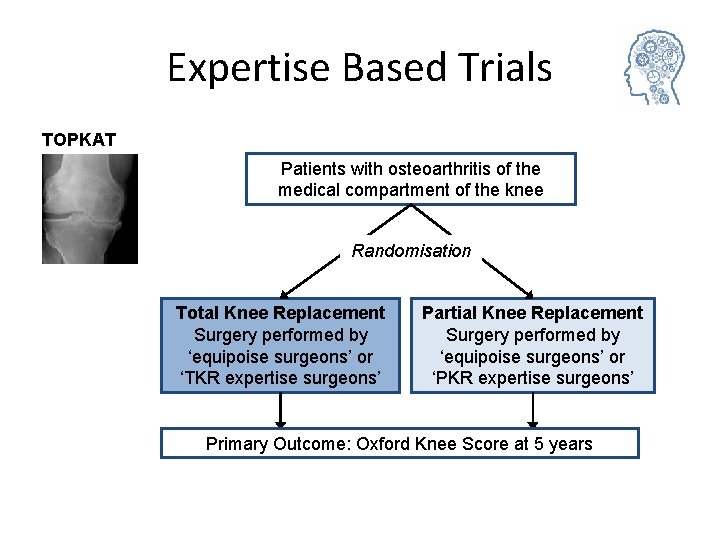
Expertise Based Trials TOPKAT Patients with osteoarthritis of the medical compartment of the knee Randomisation Total Knee Replacement Surgery performed by ‘equipoise surgeons’ or ‘TKR expertise surgeons’ Partial Knee Replacement Surgery performed by ‘equipoise surgeons’ or ‘PKR expertise surgeons’ Primary Outcome: Oxford Knee Score at 5 years

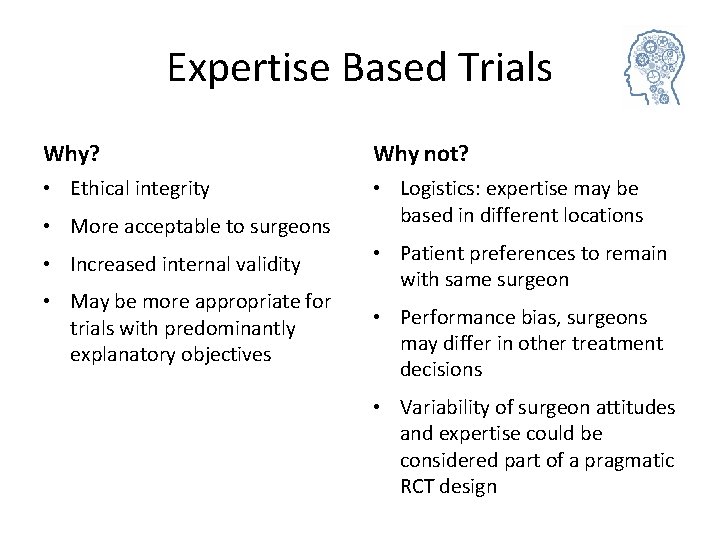
Expertise Based Trials Why? Why not? • Ethical integrity • Logistics: expertise may be based in different locations • More acceptable to surgeons • Increased internal validity • May be more appropriate for trials with predominantly explanatory objectives • Patient preferences to remain with same surgeon • Performance bias, surgeons may differ in other treatment decisions • Variability of surgeon attitudes and expertise could be considered part of a pragmatic RCT design

Summary • Many different RCT design options available – Some are necessary given experimental set-up and logistic constraints – Some are desirable to obtain greatest value for patient burden and expense of conducting a clinical trial – Opportunity to incorporate innovative designs (e. g. MAMS) • Recommendation to aim for achieving maximum efficiency for given amount of resources • Be aware of additional procedural and analytical issues arising from complex trial designs

Relevant Papers • Cook JA. The challenges faced in the design, conduct and analysis of surgical randomised controlled trials. Trials. 2009; 10: 9. • Mundi R, Chaudhry H, Mundi S, Godin K, Bhandari M. Design and execution of clinical trials in orthopaedic surgery. Bone & joint research. 2014; 3(5): 161 -8. • Perry DC, Griffin XL, Parsons N, Costa ML. Designing clinical trials in trauma surgery: overcoming research barriers. Bone & joint research. 2014; 3(4): 123 -9. • Pinkney TD, Morton DG. Novel approaches to surgical trials and the assessment of new surgical technologies. The British journal of surgery. 2015; 102(2): e 10 -1. • Scholtes VA, Nijman TH, van Beers L, Devereaux PJ, Poolman RW. Emerging designs in orthopaedics: expertise-based randomized controlled trials. The Journal of bone and joint surgery American volume. 2012; 94 Suppl 1: 24 -8. • Wason JM, Jaki T. Optimal design of multi-arm multi-stage trials. Statistics in medicine. 2012; 31(30): 4269 -79.

References for Example Studies • • • Attard G, Sydes MR, Mason MD, Clarke NW, Aebersold D, de Bono JS, et al. Combining enzalutamide with abiraterone, prednisone, androgen deprivation therapy in the STAMPEDE trial. European urology. 2014; 66(5): 799 -802. Beard D, Price A, Cook J, Fitzpatrick R, Carr A, Campbell M, et al. Total or Partial Knee Arthroplasty Trial - TOPKAT: study protocol for a randomised controlled trial. Trials. 2013; 14: 292. Bhandari M, Devereaux PJ, Einhorn TA, Thabane L, Schemitsch EH, Koval KJ, et al. Hip fracture evaluation with alternatives of total hip arthroplasty versus hemiarthroplasty (HEALTH): protocol for a multicentre randomised trial. BMJ open. 2015; 5(2): e 006263. Grieve AP, Krams M. ASTIN: a Bayesian adaptive dose-response trial in acute stroke. Clinical trials (London, England). 2005; 2(4): 340 -51; discussion 52 -8, 64 -78. Holm B, Husted H, Kehlet H, Bandholm T. Effect of knee joint icing on knee extension strength and knee pain early after total knee arthroplasty: a randomized cross-over study. Clinical rehabilitation. 2012; 26(8): 716 -23 Klaber Moffett JA, Jackson DA, Richmond S, Hahn S, Coulton S, Farrin A, et al. Randomised trial of a brief physiotherapy intervention compared with usual physiotherapy for neck pain patients: outcomes and patients' preference. BMJ (Clinical research ed). 2005; 330(7482): 75.

References for Example Studies • • • Peel TN, Cheng AC, Buising KL, Dowsey MM, Choong PF. Alcoholic Chlorhexidine or Alcoholic Iodine Skin Antisepsis (ACAISA): protocol for cluster randomised controlled trial of surgical skin preparation for the prevention of superficial wound complications in prosthetic hip and knee replacement surgery. BMJ open. 2014; 4(5): e 005424. Rangan A, Handoll H, Brealey S, Jefferson L, Keding A, Martin BC, et al. Surgical vs nonsurgical treatment of adults with displaced fractures of the proximal humerus: the PROFHER randomized clinical trial. Jama. 2015; 313(10): 1037 -47. Robson SC, Kelly T, Howel D, Deverill M, Hewison J, Lie ML, et al. Randomised preference trial of medical versus surgical termination of pregnancy less than 14 weeks' gestation (TOPS). Health technology assessment (Winchester, England). 2009; 13(53): 1 -124, iii-iv. So-Osman C, Nelissen RG, Koopman-van Gemert AW, Kluyver E, Poll RG, Onstenk R, et al. Patient blood management in elective total hip- and knee-replacement surgery (Part 1): a randomized controlled trial on erythropoietin and blood salvage as transfusion alternatives using a restrictive transfusion policy in erythropoietineligible patients. Anesthesiology. 2014; 120(4): 839 -51. Voorn VM, Marang-van de Mheen PJ, So-Osman C, Kaptein AA, van der Hout A, van den Akker-van Marle ME, et al. De-implementation of expensive blood saving measures in hip and knee arthroplasties: study protocol for the LISBOA-II cluster randomized trial. Implementation science : IS. 2014; 9: 48.

Many thanks for listening. Any Questions? BOA Orthopaedic Surgery Research Centre