Complex Phase Diagram construction using Free Energy vs










- Slides: 32

Complex Phase Diagram construction using Free Energy vs. Composition Curves • To construct free energy vs. composition curves given a complex phase diagram and vice versa. Learning Objectives : After going through this learning aid , user will be able to: § Construct a phase diagram given free energy vs. composition curves at different temperatures. § Draw the schematic free energy vs. composition curves at different temperatures given the phase diagrams. § State and interpret the Gibbs Phase Rule. Author: Kartikay Agarwal 10 D 110030 UG sophomore year Dept. of ME & MS Mentor: Prof. M. P. Gururajan Dept. of ME & MS Shrey Singh 10 D 110010 UG sophomore year Dept. of ME & MS

Glossary Phase Diagram - Phase Diagrams are charts which give the information regarding the equilibrium phases in a system under the given thermodynamic constraints. Gibbs Phase Rule – At constant pressure P+F-C=1 where C=no. of components, F=no. of Degrees of freedom, P=no of phases. Eutectic Point - The point at which liquid phase tranforms into two solid phases. Peritactic Point – The point at which a liquid and a solid phase transforms into another solid phase. Eutectoid Point - The point at which solid phase tranforms into two solid phases.

Explanation Phase Diagrams are a way of cataloguing the information regarding the equilibrium phases in a system under the given thermodynamic constraints. In this learning object, we will consider a system of a binary alloy at constant pressure and different temperatures. So the phase diagram is a plot of composition vs. temperature with the equilibrium phases marked at different combinations of these two parameters. Specifically in this animation we consider a system that shows the existence of one , two and three at any temperature and composition. We will show that the equilibrium phases are a result of the minimization of the free energy of the system.

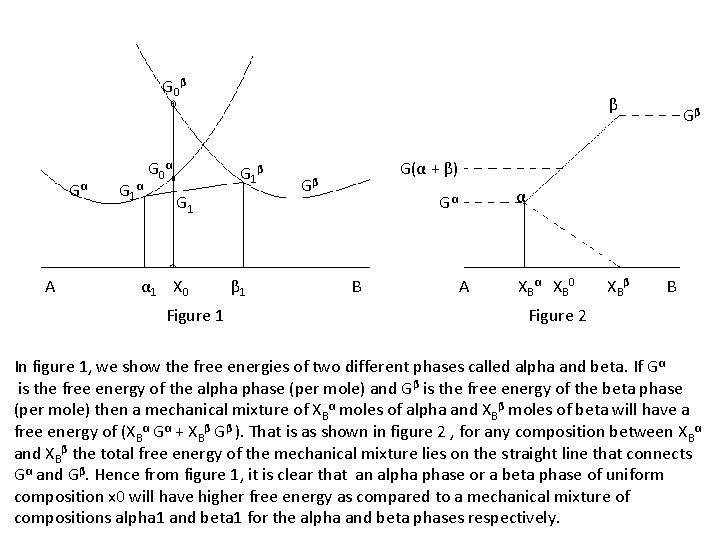
G 0β Gα A G 1α G 0α β G 1 α 1 X 0 Figure 1 β 1 Gβ G(α + β) Gβ α Gα B A X B α XB 0 XB β B Figure 2 In figure 1, we show the free energies of two different phases called alpha and beta. If Gα is the free energy of the alpha phase (per mole) and Gβ is the free energy of the beta phase (per mole) then a mechanical mixture of XBα moles of alpha and XBβ moles of beta will have a free energy of (XBα Gα + XBβ Gβ ). That is as shown in figure 2 , for any composition between XBα and XBβ the total free energy of the mechanical mixture lies on the straight line that connects Gα and Gβ. Hence from figure 1, it is clear that an alpha phase or a beta phase of uniform composition x 0 will have higher free energy as compared to a mechanical mixture of compositions alpha 1 and beta 1 for the alpha and beta phases respectively.

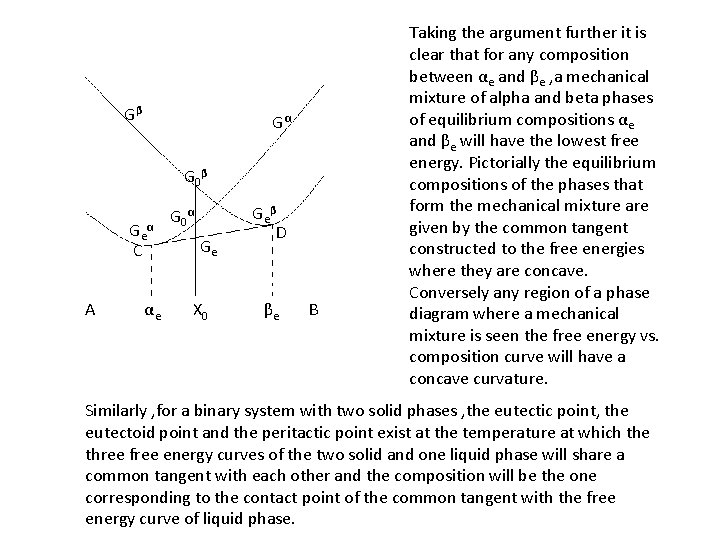
Gβ Gα G 0β Geα C A αe G 0α Ge X 0 Geβ D βe B Taking the argument further it is clear that for any composition between αe and βe , a mechanical mixture of alpha and beta phases of equilibrium compositions αe and βe will have the lowest free energy. Pictorially the equilibrium compositions of the phases that form the mechanical mixture are given by the common tangent constructed to the free energies where they are concave. Conversely any region of a phase diagram where a mechanical mixture is seen the free energy vs. composition curve will have a concave curvature. Similarly , for a binary system with two solid phases , the eutectic point, the eutectoid point and the peritactic point exist at the temperature at which the three free energy curves of the two solid and one liquid phase will share a common tangent with each other and the composition will be the one corresponding to the contact point of the common tangent with the free energy curve of liquid phase.

Slide 1 Introduction Slide 2 Glossary Tab 03 Tab 04 Tab 05 Tab 06 Tab 07 Binary Phase Diagrams Instructions/ Working area Credits

Action/Description Show the animation description and the learning objectives from slide 1. Show the glossary from slide 2 Audio narration Text to be displayed

Slide 3, 4 and 5 Introduction Glossary Explanation Tab 04 Tab 05 Tab 06 Tab 07 Binary Phase Diagrams Instructions/ Working area Credits

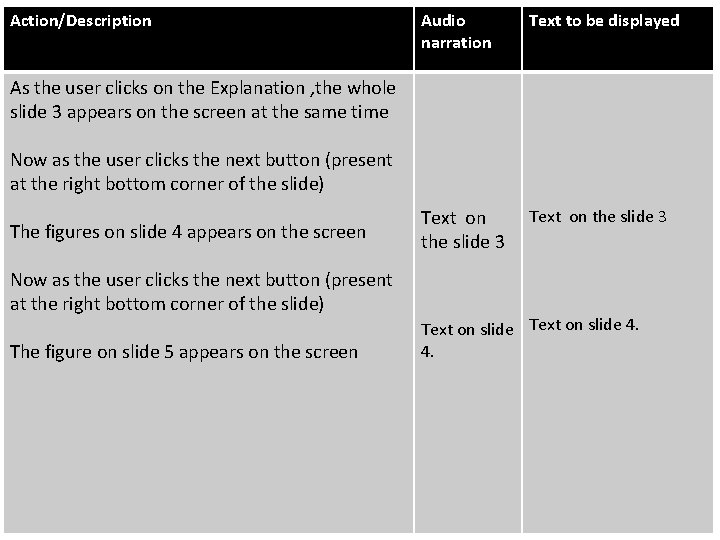
Action/Description Audio narration Text to be displayed Text on the slide 3 As the user clicks on the Explanation , the whole slide 3 appears on the screen at the same time Now as the user clicks the next button (present at the right bottom corner of the slide) The figures on slide 4 appears on the screen Now as the user clicks the next button (present at the right bottom corner of the slide) The figure on slide 5 appears on the screen Text on slide 4. 4.

Next slide Introduction Glossary Explanation Animation Tab 05 Tab 06 Tab 07 Binary Phase Diagrams Instructions/ Working area Credits

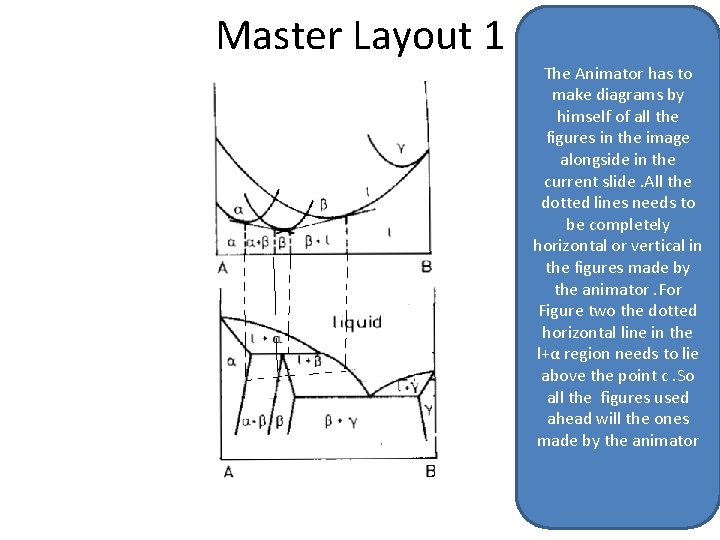
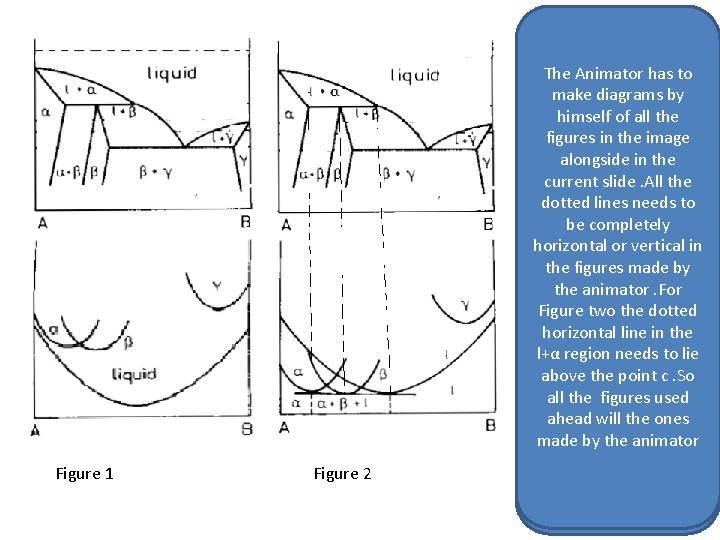
Master Layout 1 The Animator has to make diagrams by Figure himself. A of all the figures in the image alongside in the current slide. All the dotted lines needs to be completely horizontal or vertical in the figures made by the animator. For Figure two the dotted horizontal line in the l+αFigure regionb needs to lie above the point c. So all the figures used ahead will the ones made by the animator

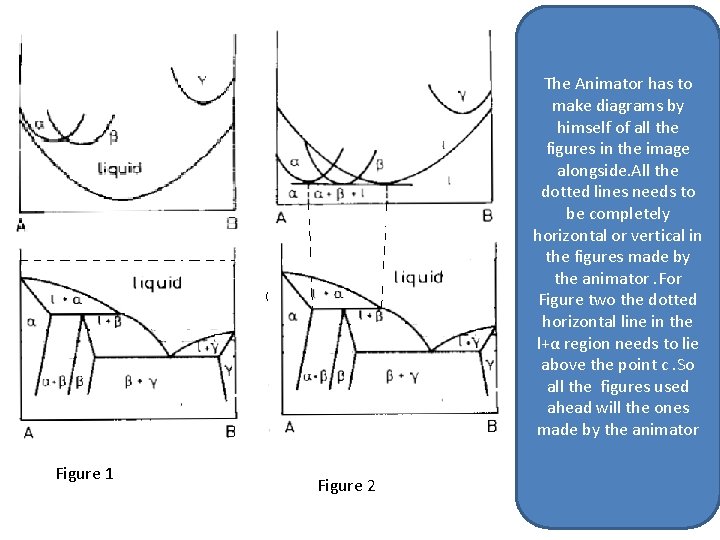
The Animator has to make diagrams by himself of all the figures in the image alongside. All the dotted lines needs to be completely horizontal or vertical in the figures made by the animator. For Figure two the dotted horizontal line in the l+α region needs to lie above the point c. So all the figures used ahead will the ones made by the animator C Figure 1 Figure 2

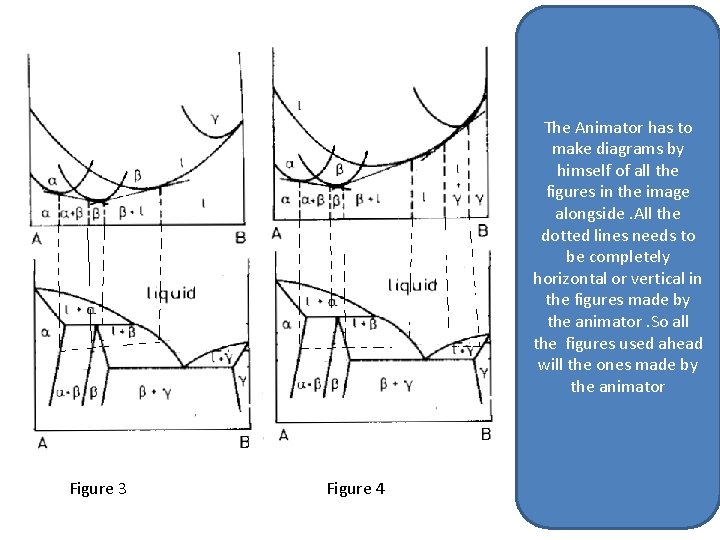
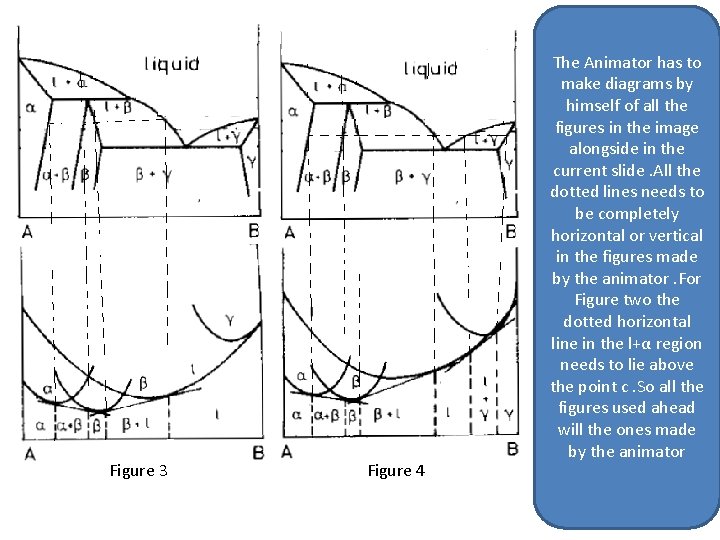
The Animator has to make diagrams by himself of all the figures in the image alongside. All the dotted lines needs to be completely horizontal or vertical in the figures made by the animator. So all the figures used ahead will the ones made by the animator Figure 3 Figure 4

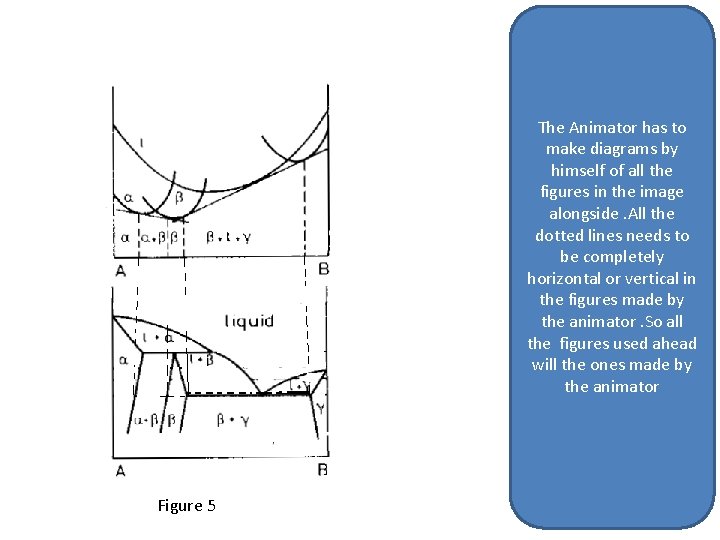
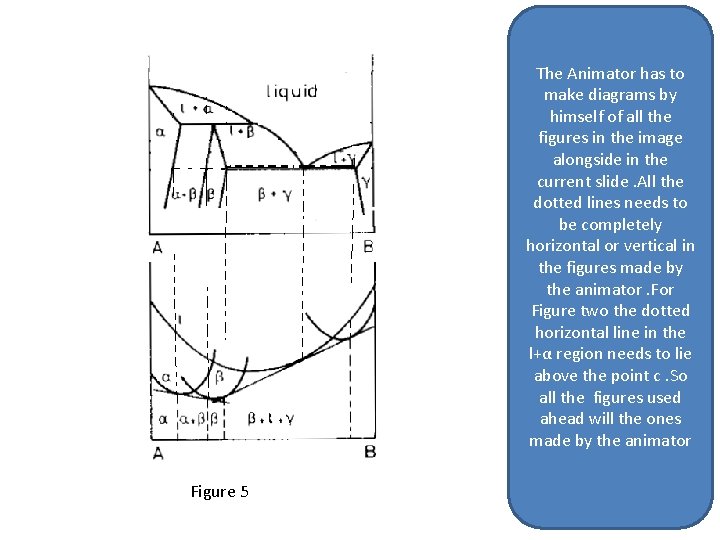
The Animator has to make diagrams by himself of all the figures in the image alongside. All the dotted lines needs to be completely horizontal or vertical in the figures made by the animator. So all the figures used ahead will the ones made by the animator Figure 5

displayed The Slide Master Layout 1 will appear on the screen in this tab but the figure in the layout will be figure 1 instead of what is shown in the master layout slide The system will exist in liquid phase until melting temperature of pure A is reached. As soon as melting point of A is reached , a mixture of liquid and α phase will exists in the region between the points where common tangent touches the two free energy curves Same as Audio Narration The figure will change from figure 1 to 2(figure 1 and 2 needs to be made by the animator as been described in the previous slide) As the eutectioid temperature is reached i. e. , the temperature at which there will be common tangent to the three free energy curves there will be a mechanical mixture of all the three phases(liquid , α & β) at the eutectoid composition Same as Audio Narration Similarly, figure will change from 2 to 3. As the temperature is further decreased there will be two common tangents in the free energy vs composition curves and hence there will be two regions in the phase diagram where a mechanical mixture of two phases will exist i. e. , of β & α and liquid & β. Same as Audio Narration

Action/Description Audio narration Text to be displayed Figure A will change from 3 to 4. As the temperature is further decreased there will be three common tangents in the free energy vs composition curves and hence there will be three regions in the phase diagram where a mechanical mixture of two phases will exist i. e. , of β & α and liquid & β and liquid + ϒ. Same as Audio Narration Now the figure will change from figure 4 to figure 5 As soon as the eutectic temperature is reached, there will be a mechanical mixture of three phases l, beta and gama at the composition where the common tangent to the three free energy curves of the same touches the liquid free energy curve Also there exists a mechanical mixture of alpha and beta in the region of common tangent between alpha and beta free energy curves Same as Audio Narration During transition curve α & β & ϒ will move downward and l will move upward gradually relative to each other. Next Button Appears on the Right Bottom Click on Next for next animation

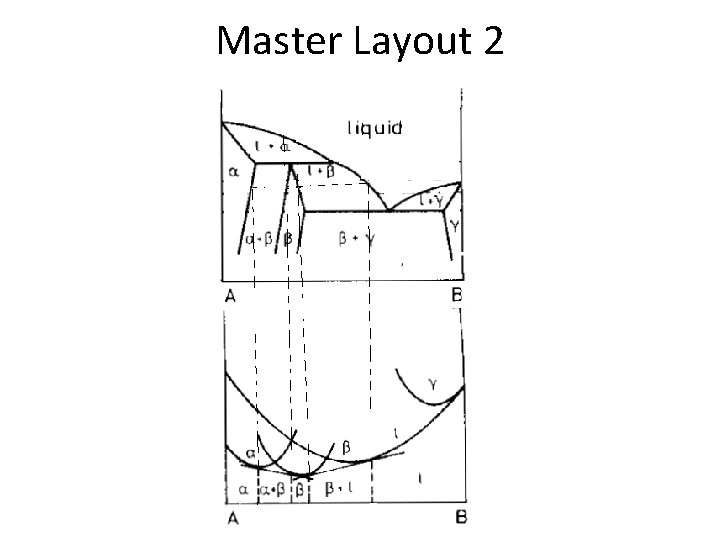
Master Layout 2

The Animator has to to make diagrams by by himself of of all the figures in in the image alongside in in the current slide. All the dotted lines needs to to be be completely horizontal or or vertical in in the figures made by by the animator. For Figure two the dotted horizontal line in in the l+α region needs to to lie above the point cc. So all the figures used ahead will the ones made by by the animator Figure 1 Figure 2

Figure 3 Figure 4 The Animator has to make diagrams by himself of all the figures in the image alongside in the current slide. All the dotted lines needs to be completely horizontal or vertical in the figures made by the animator. For Figure two the dotted horizontal line in the l+α region needs to lie above the point c. So all the figures used ahead will the ones made by the animator

The Animator has to make diagrams by himself of all the figures in the image alongside in the current slide. All the dotted lines needs to be completely horizontal or vertical in the figures made by the animator. For Figure two the dotted horizontal line in the l+α region needs to lie above the point c. So all the figures used ahead will the ones made by the animator Figure 5

displayed The Slide Master Layout 1 will appear on the screen in this tab but the figure in the layout will be figure 1 instead of what is shown in the master layout slide The figure will change from figure 1 to 2(figure 1 and 2 needs to be made by the animator as been described in the previous slide) Similarly, figure will change from 2 to 3. For region above melting temperature of A, No of Phases is just 1 i. e. , solid and components are 2 so by Gibbs Phase Rule degrees of freedom will be 1 which means that we can obtain a particular composition of liquid phase by varying temperature and composition simultaneously. At the peritactic temperature and composition , three phases exists which gives out degrees of freedom to be 0 from Gibbs Phase Rule which means that for a system at constant pressure, peritactic point exists at a fixed temperature and composition. The region where exists a mechanical mixture of two phases (β+α or l+β), F comes out to be 1 which means that in order to obtain a particular composition of a phase we can chose either one of temperature or composition independently but the other one needs to be fixed. Same as audio narration

Action/Description Audio narration Figure A will change from 3 to 4. On going below melting Same as temperature of B, There will audio three regions with 1 degree narration of freedom(β+α or l+β or l+ϒ) Now the figure will change from figure 4 to figure 5 During transition curve α & β & ϒ will move downward and l will move upward gradually relative to each other. Next Button Appears on the Right Bottom At the Eutectic point and composition , three phases exists which gives out degrees of freedom to be 0 from gibbs phase rule which means that for a system at constant pressure , eutectic point exists at a fixed temperature and composition. Although there Will a region where the degree will be two representing a mixture of β+α. Click on Next for next slide. Text to be displayed Same as audio narration.

Next Slide Introduction Glossary Explanation Animation Questionnaire Tab 06 Tab 07 Binary Phase Diagrams Instructions/ Working area Credits

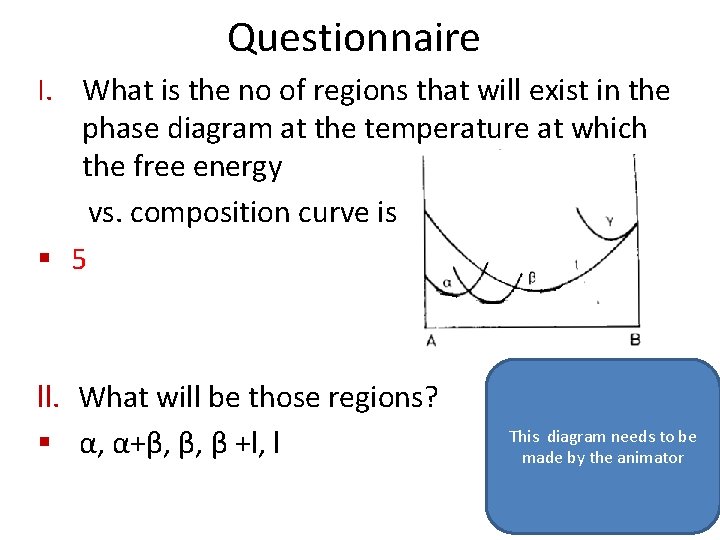
Questionnaire I. What is the no of regions that will exist in the phase diagram at the temperature at which the free energy vs. composition curve is § 5 ll. What will be those regions? § α, α+β, β, β +l, l This diagram needs to be made by the animator

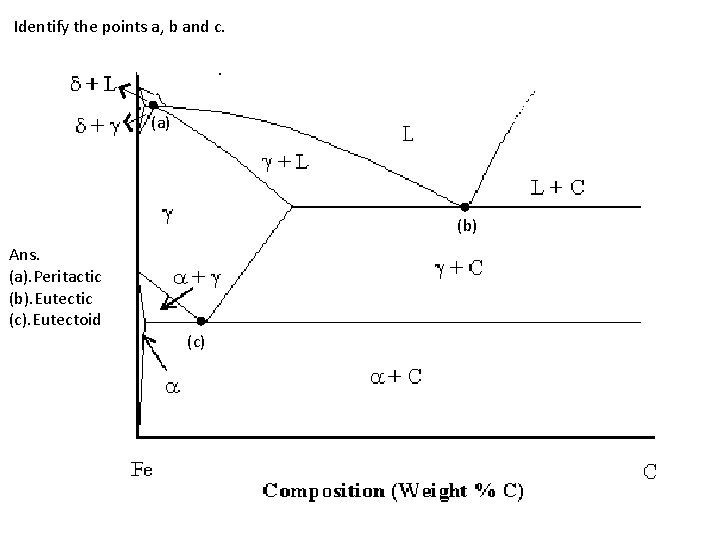
Identify the points a, b and c. (a) (b) Ans. (a). Peritactic (b). Eutectic (c). Eutectoid (c)

Action/Description Audio narration As the user clicks on the Questionnaire tab , the questions written in black color appear on screen along with the figure(to be made by the animator as described in the previous slide) at the same time. Then on selecting the answers tab made at the bottom of screen, the answer, i. e. text written in red color appears in the same format as shown in previous slide. Same as as on on the slide Text to be displayed

Next Slide Introduction Glossary Explanation Activity Area Questionnaire Summary Tab 07 Binary Phase Diagrams Instructions/ Working area Credits

Summary By the interpretation of free energy vs. composition curves , we can estimate the phase diagram and also the free energy vs. composition curves can be estimated on the basis of phase diagrams. Free energy vs. composition curves explain the reason behind the existence of multiple phases at different temperatures and compositions. The phase or a mixture of phases with the lowest free energy will exist at all combination of temperatures and compositions in order to attain equilibrium.

Action/Description Audio narration Show the summary from previous slide Same as on the slide Text to be displayed

Next Slide Introduction Glossary Explanation Activity Area Questionnaire Summary Further Reading Binary Phase Diagrams Instructions/ Working area Credits

Further Reading Books • Phase Transformations in Metals and Alloys by David A Porter K. E. Easterling Published by Chapman & Hall, 2 -6 Bormdary Row, London SEl 8 HN, UK Links • http: //www. sv. vt. edu/classes/MSE 2094_Note. Book/96 Class Proj/analytic/anmeth. html • http: //www. matter. org. uk/matscicdrom/manual/td. html • http: //en. wikipedia. org/wiki/Phase_diagram

Action/Description Audio narration Show further reading from previous slide Same as on the slide Text to be displayed