Complete combustion In excess oxygen short chain alkanes
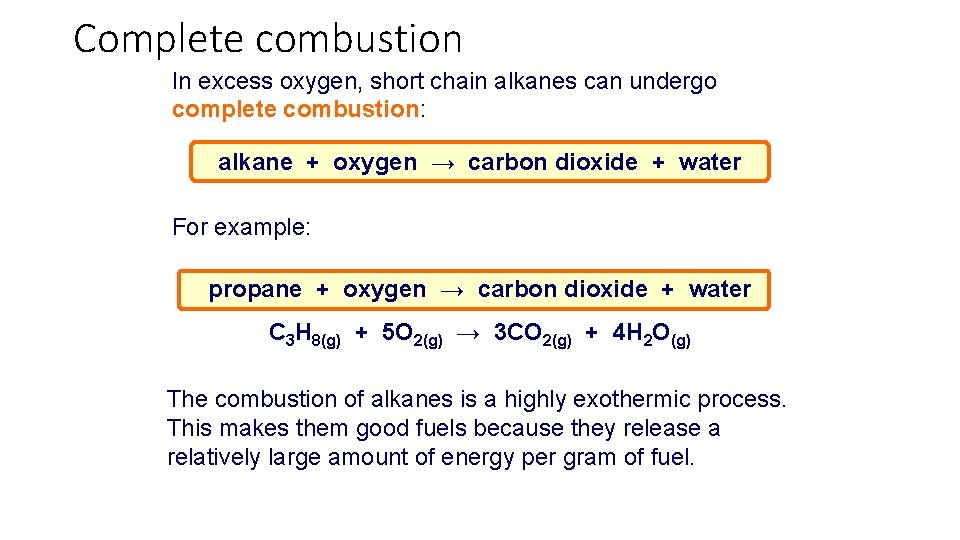
Complete combustion In excess oxygen, short chain alkanes can undergo complete combustion: alkane + oxygen → carbon dioxide + water For example: propane + oxygen → carbon dioxide + water C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(g) The combustion of alkanes is a highly exothermic process. This makes them good fuels because they release a relatively large amount of energy per gram of fuel.
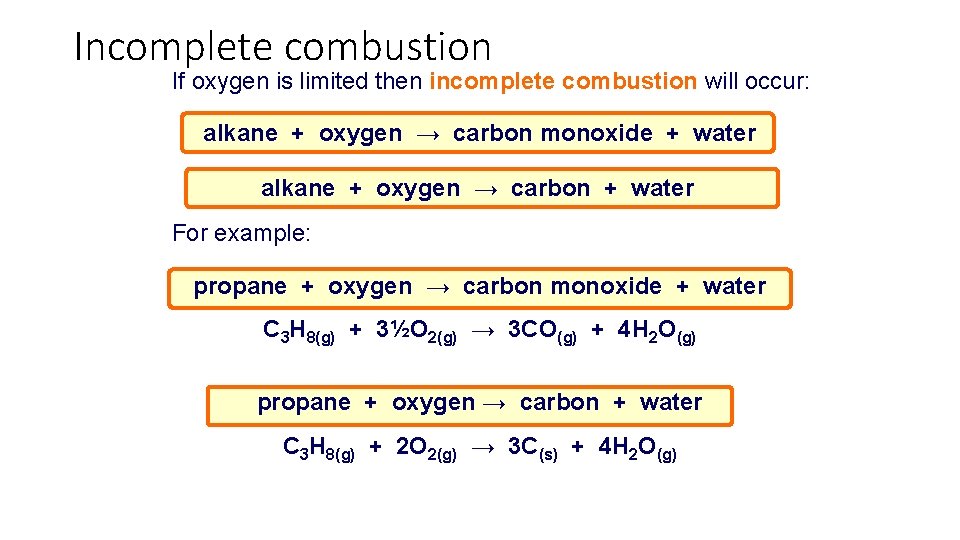
Incomplete combustion If oxygen is limited then incomplete combustion will occur: alkane + oxygen → carbon monoxide + water alkane + oxygen → carbon + water For example: propane + oxygen → carbon monoxide + water C 3 H 8(g) + 3½O 2(g) → 3 CO(g) + 4 H 2 O(g) propane + oxygen → carbon + water C 3 H 8(g) + 2 O 2(g) → 3 C(s) + 4 H 2 O(g)
Functional Groups The grouping of hydrocarbon compounds into families (alkanes, alkenes, alkynes, alcohols, carboxylic acids and non-branched esters) based upon similarities in their physical and chemical properties including general formulas, their representations (structural formulas, condensed formulas, Lewis structures), naming according to IUPAC systematic nomenclature (limited to non-cyclic compounds up to C 10, and structural isomers up to C 7) and uses based upon properties
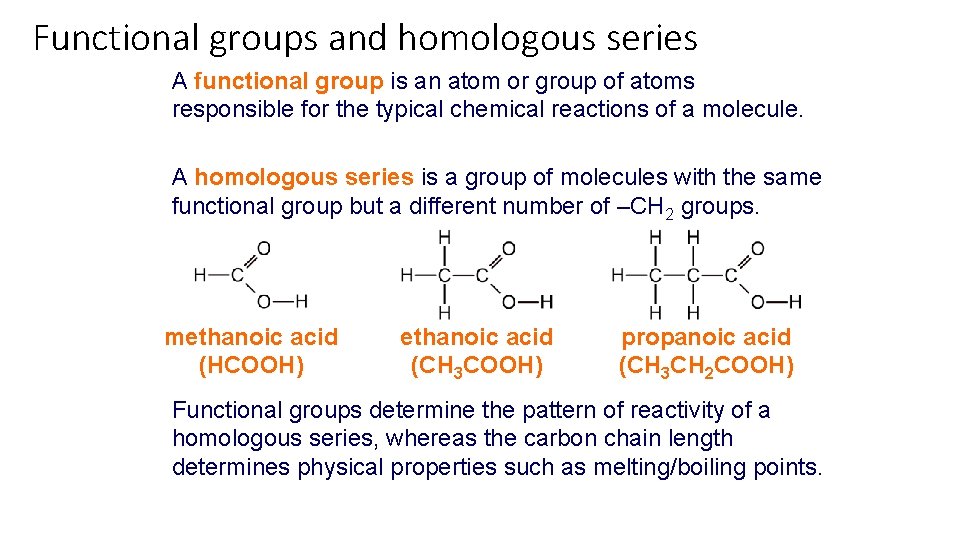
Functional groups and homologous series A functional group is an atom or group of atoms responsible for the typical chemical reactions of a molecule. A homologous series is a group of molecules with the same functional group but a different number of –CH 2 groups. methanoic acid (HCOOH) ethanoic acid (CH 3 COOH) propanoic acid (CH 3 CH 2 COOH) Functional groups determine the pattern of reactivity of a homologous series, whereas the carbon chain length determines physical properties such as melting/boiling points.
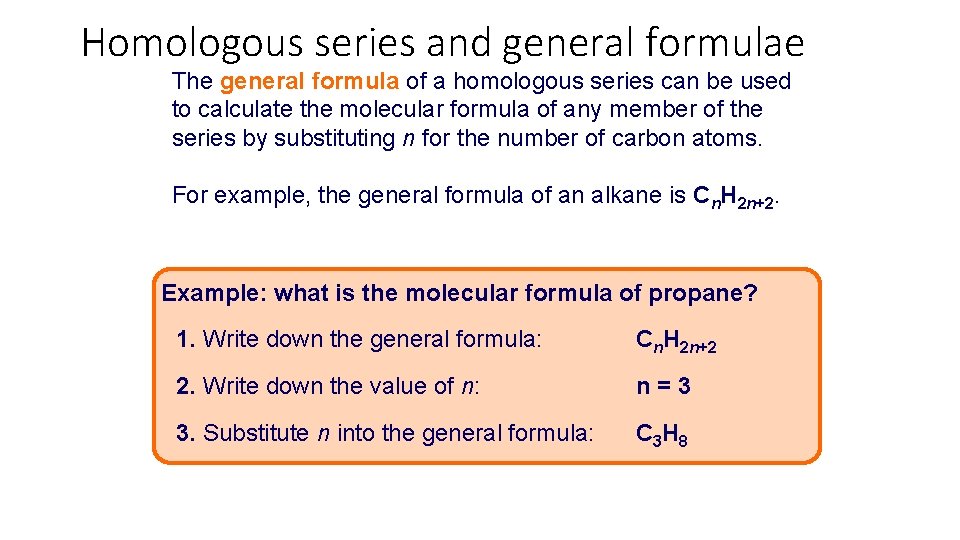
Homologous series and general formulae The general formula of a homologous series can be used to calculate the molecular formula of any member of the series by substituting n for the number of carbon atoms. For example, the general formula of an alkane is Cn. H 2 n+2. Example: what is the molecular formula of propane? 1. Write down the general formula: Cn. H 2 n+2 2. Write down the value of n: n=3 3. Substitute n into the general formula: C 3 H 8
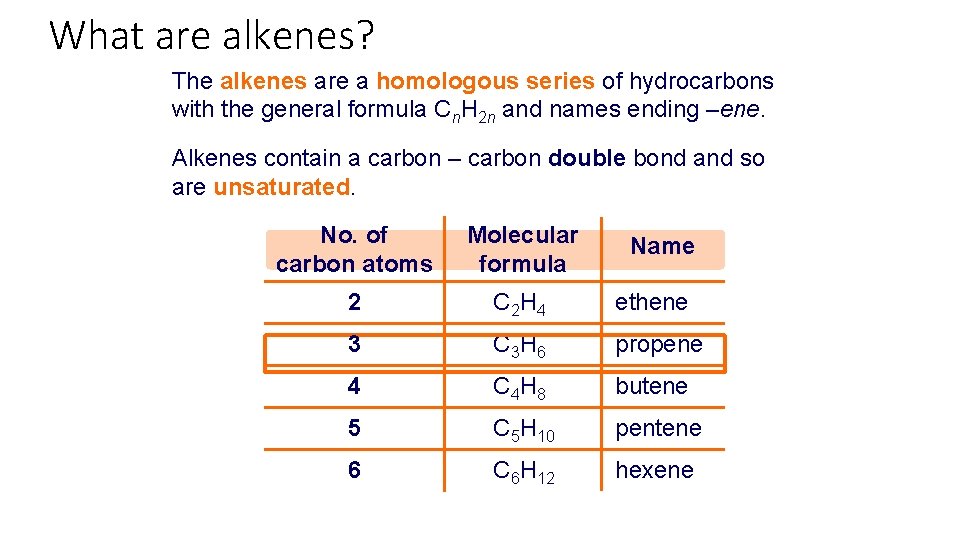
What are alkenes? The alkenes are a homologous series of hydrocarbons with the general formula Cn. H 2 n and names ending –ene. Alkenes contain a carbon – carbon double bond and so are unsaturated. No. of carbon atoms Molecular formula 2 C 2 H 4 ethene 3 C 3 H 6 propene 4 C 4 H 8 butene 5 C 5 H 10 pentene 6 C 6 H 12 hexene Name
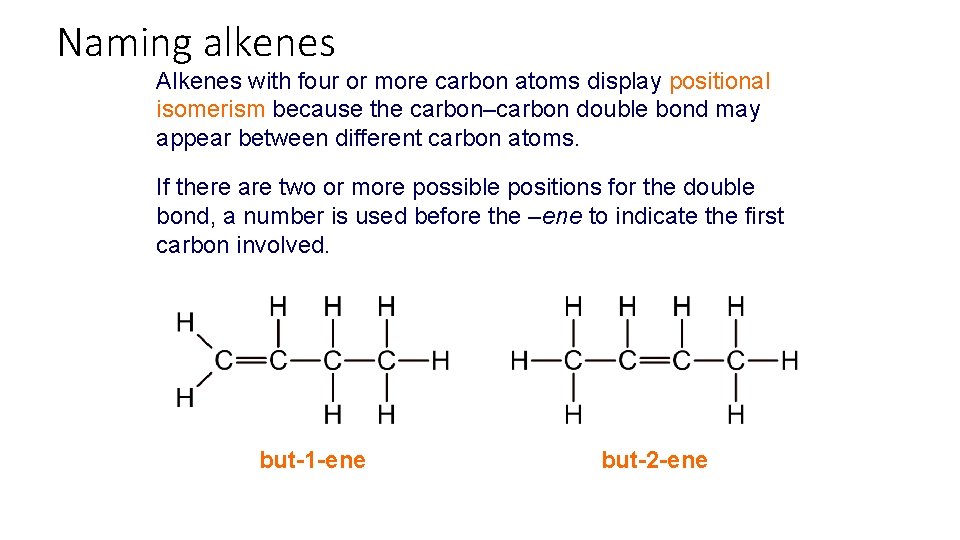
Naming alkenes Alkenes with four or more carbon atoms display positional isomerism because the carbon–carbon double bond may appear between different carbon atoms. If there are two or more possible positions for the double bond, a number is used before the –ene to indicate the first carbon involved. but-1 -ene but-2 -ene

Reactions with alkenes • We rely on alkenes for many things. • Ethene is the most common alkene used • Hydration (adding steam)- turns it into an alcohol • Polymerisation - monomers undergo polymerisation to form polymers (plastics) • Hydrogenation (adding Hydrogen)- Used in the manufacturing of soft lipids like margarine and vegetable oils • Halogenation (adding a halogen)

Testing for alkenes The presence of unsaturation (a carbon– carbon double bond) can be detected using bromine water, a red/orange coloured solution of bromine. A few drops of bromine water are added to the test liquid and shaken. If a carbon–carbon double bond is present, the bromine adds across it and the solution turns colourless.
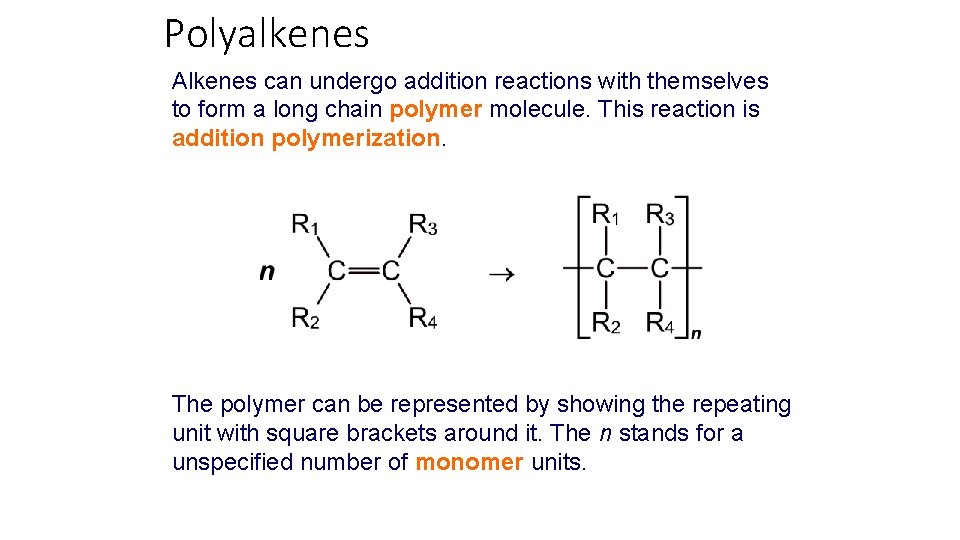
Polyalkenes Alkenes can undergo addition reactions with themselves to form a long chain polymer molecule. This reaction is addition polymerization. The polymer can be represented by showing the repeating unit with square brackets around it. The n stands for a unspecified number of monomer units.
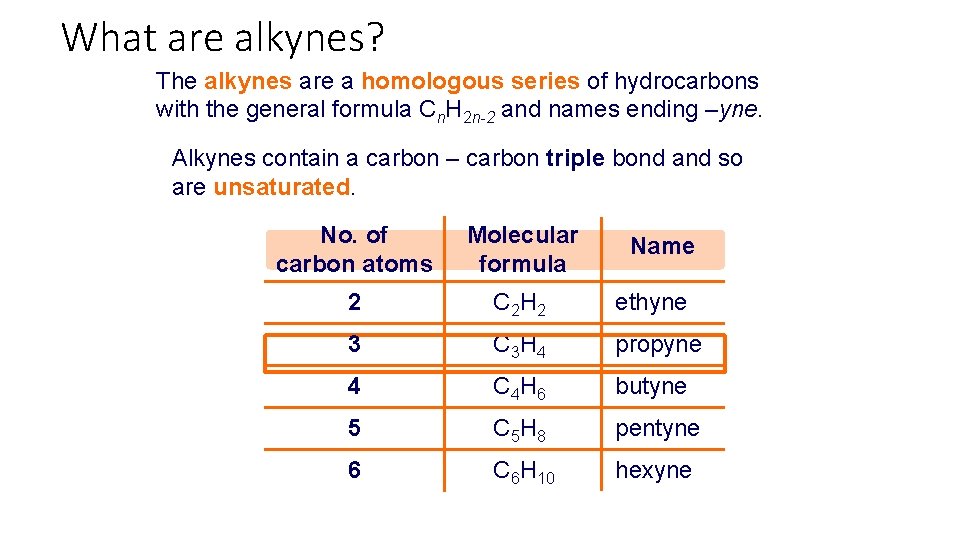
What are alkynes? The alkynes are a homologous series of hydrocarbons with the general formula Cn. H 2 n-2 and names ending –yne. Alkynes contain a carbon – carbon triple bond and so are unsaturated. No. of carbon atoms Molecular formula 2 C 2 H 2 ethyne 3 C 3 H 4 propyne 4 C 4 H 6 butyne 5 C 5 H 8 pentyne 6 C 6 H 10 hexyne Name
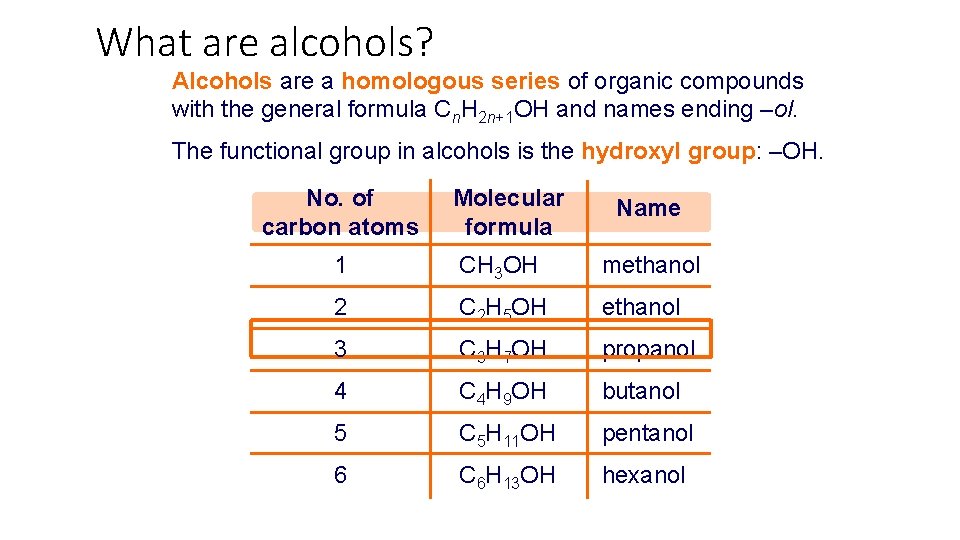
What are alcohols? Alcohols are a homologous series of organic compounds with the general formula Cn. H 2 n+1 OH and names ending –ol. The functional group in alcohols is the hydroxyl group: –OH. No. of carbon atoms Molecular formula Name 1 CH 3 OH methanol 2 C 2 H 5 OH ethanol 3 C 3 H 7 OH propanol 4 C 4 H 9 OH butanol 5 C 5 H 11 OH pentanol 6 C 6 H 13 OH hexanol
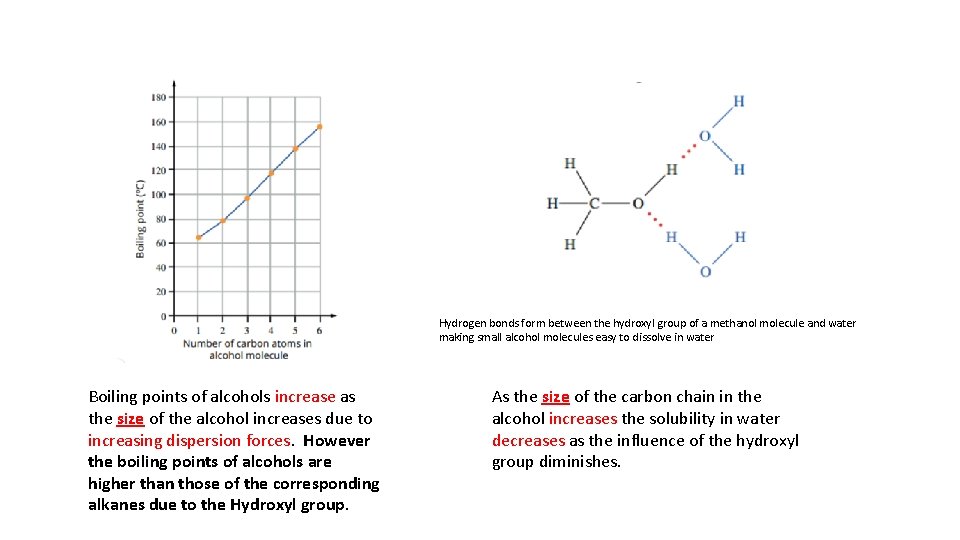
Hydrogen bonds form between the hydroxyl group of a methanol molecule and water making small alcohol molecules easy to dissolve in water Boiling points of alcohols increase as the size of the alcohol increases due to increasing dispersion forces. However the boiling points of alcohols are higher than those of the corresponding alkanes due to the Hydroxyl group. As the size of the carbon chain in the alcohol increases the solubility in water decreases as the influence of the hydroxyl group diminishes.
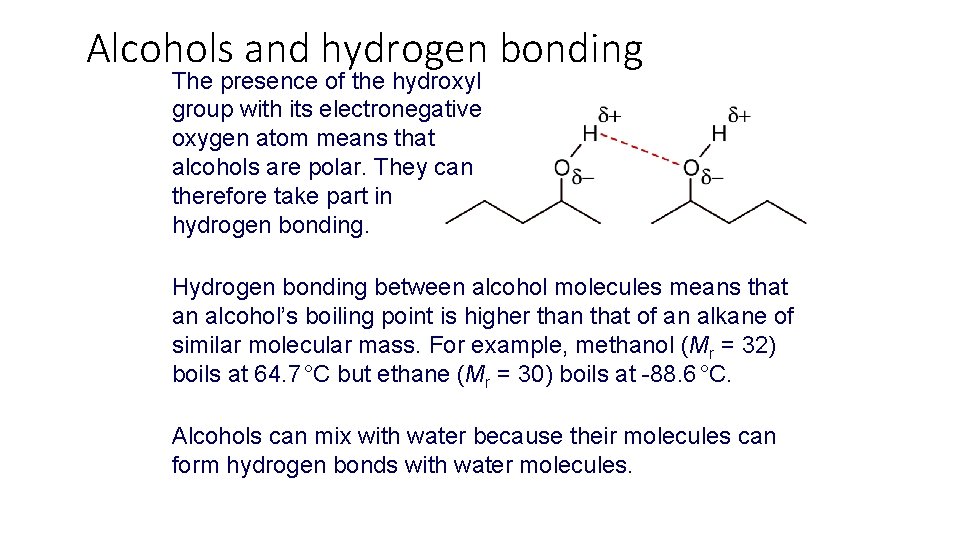
Alcohols and hydrogen bonding The presence of the hydroxyl group with its electronegative oxygen atom means that alcohols are polar. They can therefore take part in hydrogen bonding. Hydrogen bonding between alcohol molecules means that an alcohol’s boiling point is higher than that of an alkane of similar molecular mass. For example, methanol (Mr = 32) boils at 64. 7 °C but ethane (Mr = 30) boils at -88. 6 °C. Alcohols can mix with water because their molecules can form hydrogen bonds with water molecules.
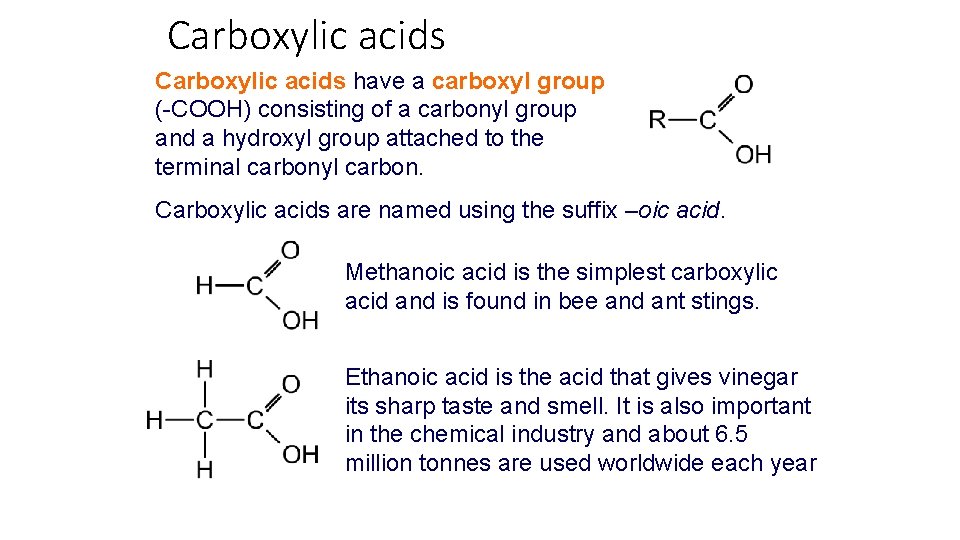
Carboxylic acids have a carboxyl group (-COOH) consisting of a carbonyl group and a hydroxyl group attached to the terminal carbonyl carbon. Carboxylic acids are named using the suffix –oic acid. Methanoic acid is the simplest carboxylic acid and is found in bee and ant stings. Ethanoic acid is the acid that gives vinegar its sharp taste and smell. It is also important in the chemical industry and about 6. 5 million tonnes are used worldwide each year
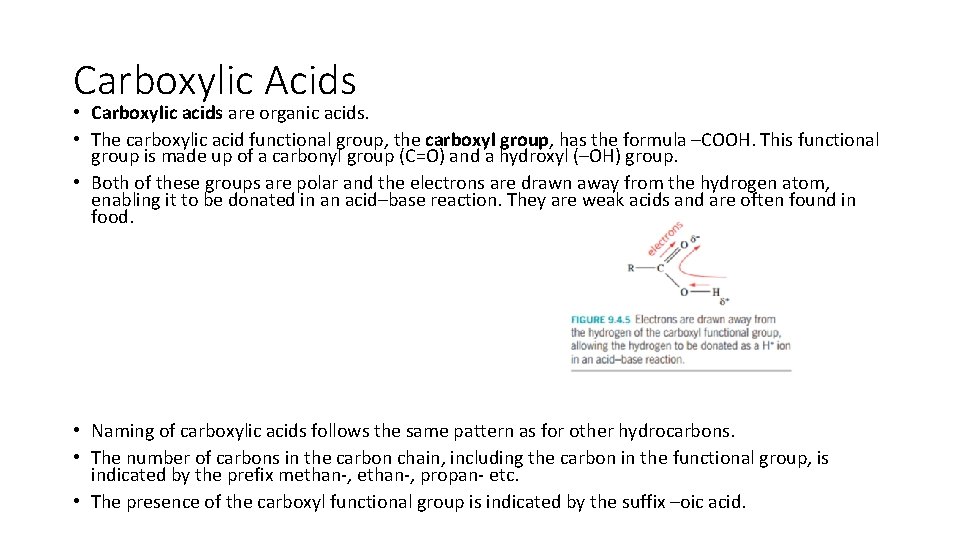
Carboxylic Acids • Carboxylic acids are organic acids. • The carboxylic acid functional group, the carboxyl group, has the formula –COOH. This functional group is made up of a carbonyl group (C=O) and a hydroxyl (–OH) group. • Both of these groups are polar and the electrons are drawn away from the hydrogen atom, enabling it to be donated in an acid–base reaction. They are weak acids and are often found in food. • Naming of carboxylic acids follows the same pattern as for other hydrocarbons. • The number of carbons in the carbon chain, including the carbon in the functional group, is indicated by the prefix methan-, propan- etc. • The presence of the carboxyl functional group is indicated by the suffix –oic acid.
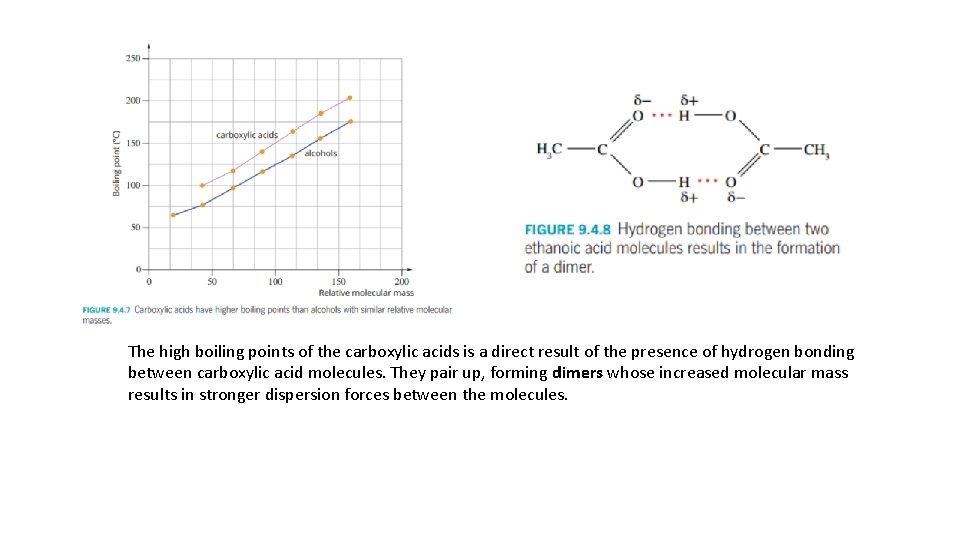
The high boiling points of the carboxylic acids is a direct result of the presence of hydrogen bonding between carboxylic acid molecules. They pair up, forming dimers whose increased molecular mass results in stronger dispersion forces between the molecules.
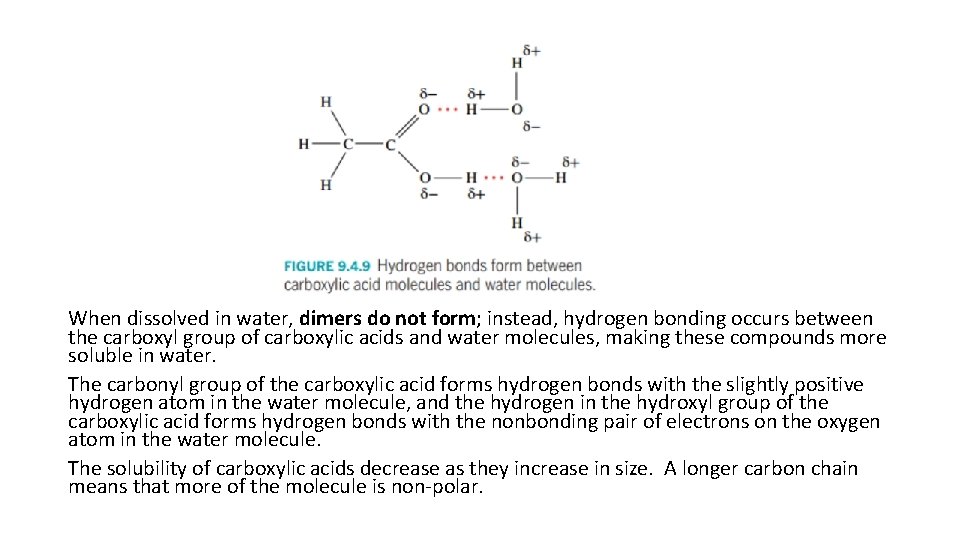
When dissolved in water, dimers do not form; instead, hydrogen bonding occurs between the carboxyl group of carboxylic acids and water molecules, making these compounds more soluble in water. The carbonyl group of the carboxylic acid forms hydrogen bonds with the slightly positive hydrogen atom in the water molecule, and the hydrogen in the hydroxyl group of the carboxylic acid forms hydrogen bonds with the nonbonding pair of electrons on the oxygen atom in the water molecule. The solubility of carboxylic acids decrease as they increase in size. A longer carbon chain means that more of the molecule is non-polar.
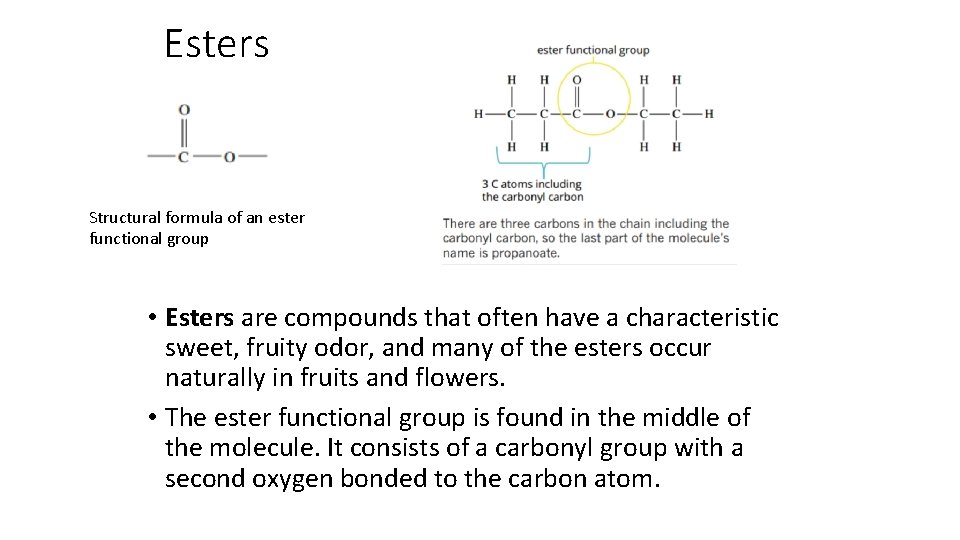
Esters Structural formula of an ester functional group • Esters are compounds that often have a characteristic sweet, fruity odor, and many of the esters occur naturally in fruits and flowers. • The ester functional group is found in the middle of the molecule. It consists of a carbonyl group with a second oxygen bonded to the carbon atom.
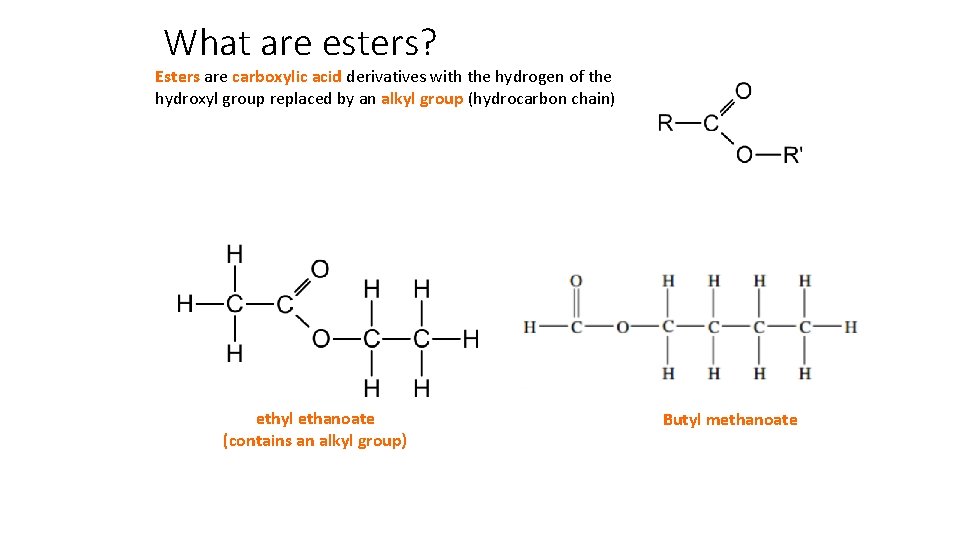
What are esters? Esters are carboxylic acid derivatives with the hydrogen of the hydroxyl group replaced by an alkyl group (hydrocarbon chain) ethyl ethanoate (contains an alkyl group) Butyl methanoate
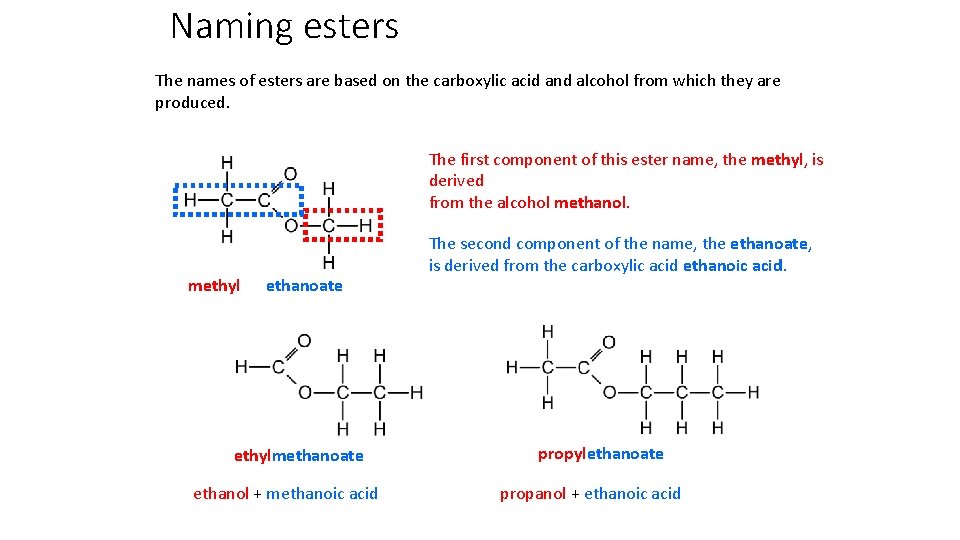
Naming esters The names of esters are based on the carboxylic acid and alcohol from which they are produced. The first component of this ester name, the methyl, is derived from the alcohol methanol. methyl ethanoate ethylmethanoate ethanol + methanoic acid The second component of the name, the ethanoate, is derived from the carboxylic acid ethanoic acid. propylethanoate propanol + ethanoic acid
- Slides: 21