Competency 2 Physical Science Make the flash cards
















- Slides: 39

Competency 2: Physical Science Make the flash cards for your areas of weakness. Use the color contrast to put information from one color on the front and the information for the other color on the back of your cards

Obj. 2 a: Chemical Equations (one card per formula) FRONT BACK Na. Cl (table salt) or (sodium chloride) H 20 (water) C 6 H 12 O 6 (sugar/glucose) O 2 (oxygen gas) CO 2 (carbon dioxide) N 2 (nitrogen gas) CH 4 (methane) O 3 (Ozone) 1 question

Obj. 2 a: Conservation of Mass Law of conservation of mass: • matter cannot be created nor destroyed, therefore the mass of the reactants must equal the mass of the products

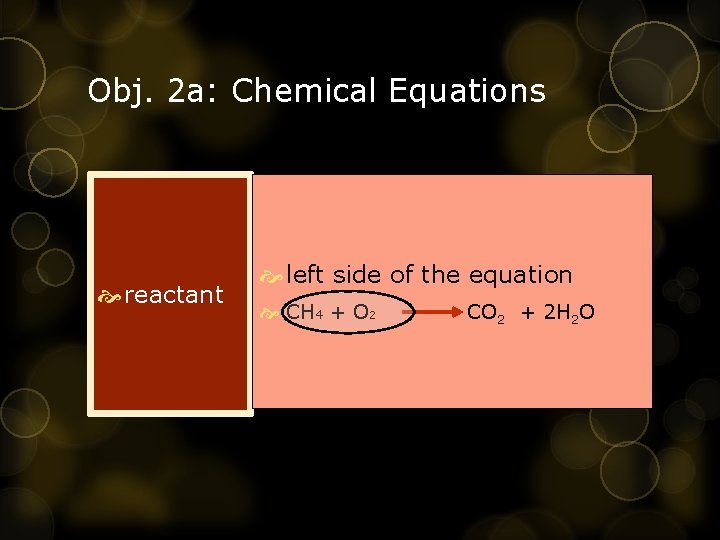
Obj. 2 a: Chemical Equations reactant left side of the equation CH 4 + O 2 CO 2 + 2 H 2 O

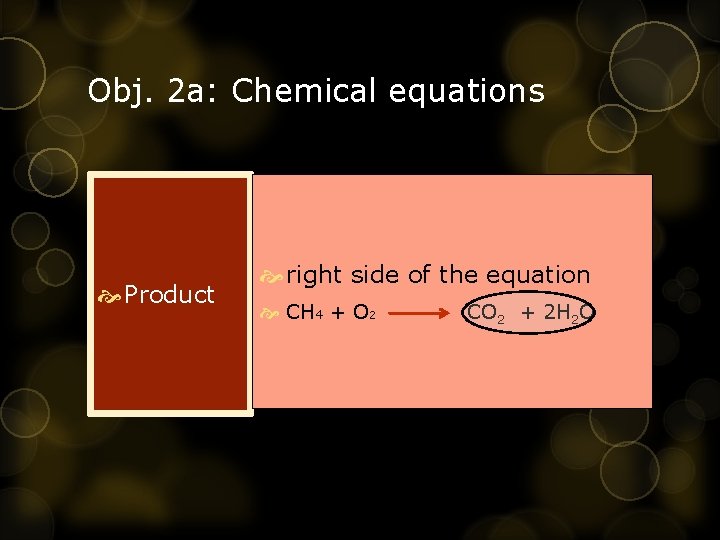
Obj. 2 a: Chemical equations Product right side of the equation CH 4 + O 2 CO 2 + 2 H 2 O

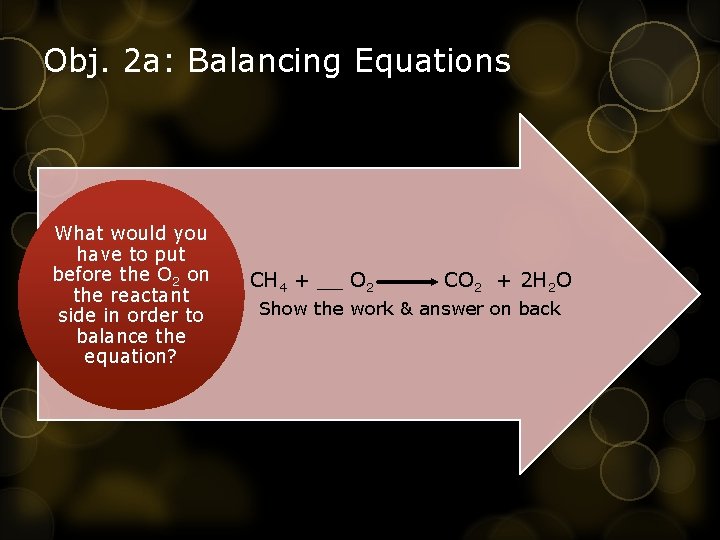
Obj. 2 a: Balancing Equations What would you have to put before the O 2 on the reactant side in order to • balance the equation? CH 4 + __ O 2 CO 2 + 2 H 2 O Show the work & answer on back

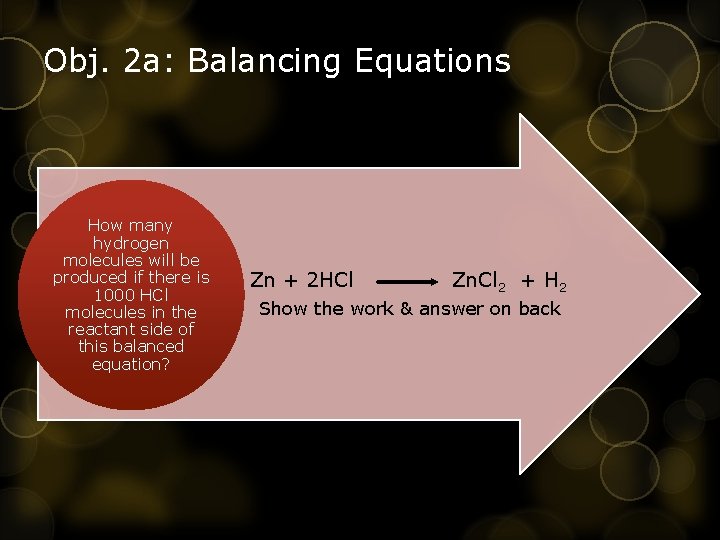
Obj. 2 a: Balancing Equations How many hydrogen molecules will be produced if there is 1000 HCl molecules in the • reactant side of this balanced equation? Zn + 2 HCl Zn. Cl 2 + H 2 Show the work & answer on back

Obj. 2 a: Balancing Equations A scientist ended up with 123 g of Zinc Chloride and 50 g of the hydrogen molecule on the product side, how much mass of Zinc was necessary for this chemical reaction to take place if you had 105 g of Hydrochloric acid Zn + 2 HCl • Zn. Cl 2 + H 2 Show the work & answer on back

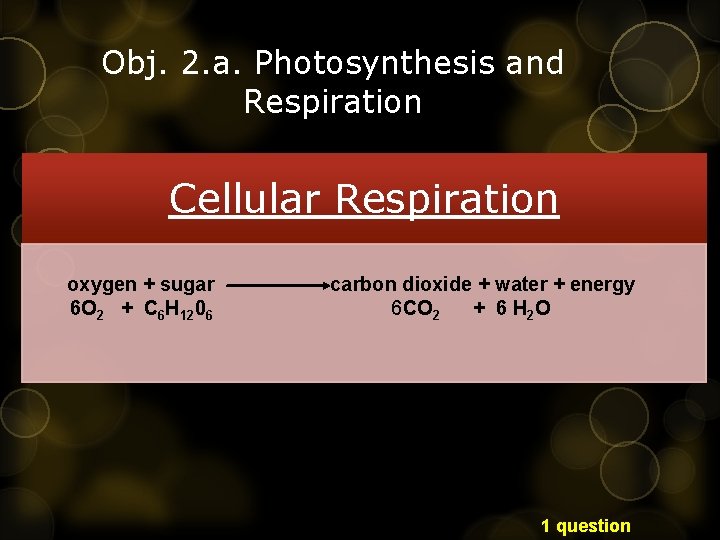
Obj. 2. a. Photosynthesis and Respiration Cellular Respiration oxygen + sugar 6 O 2 + C 6 H 1206 carbon dioxide + water + energy 6 CO 2 + 6 H 2 O 1 question

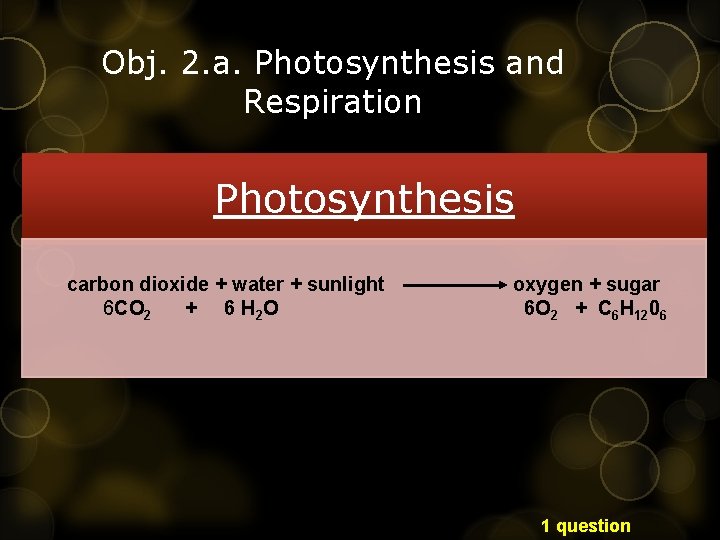
Obj. 2. a. Photosynthesis and Respiration Photosynthesis carbon dioxide + water + sunlight 6 CO 2 + 6 H 2 O oxygen + sugar 6 O 2 + C 6 H 1206 1 question

Obj. 2. b. Periodic Table Properties of nonmetals • right side of the periodic table in groups starting at the staircase • Insulator • Brittle • Low density

Obj. 2. b. Periodic Table Properties of Metals • left side of the periodic table • Malleable • Good conductors (heat & electricity) • Ductile (wires) • Luster (shiny)

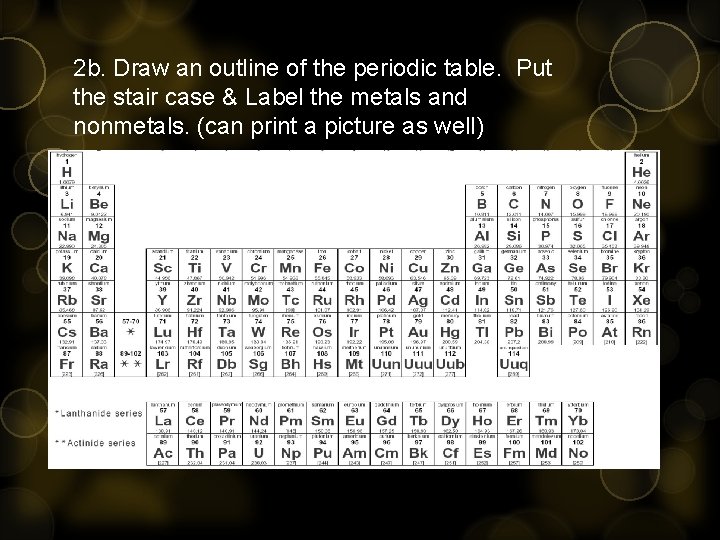
2 b. Draw an outline of the periodic table. Put the stair case & Label the metals and nonmetals. (can print a picture as well)

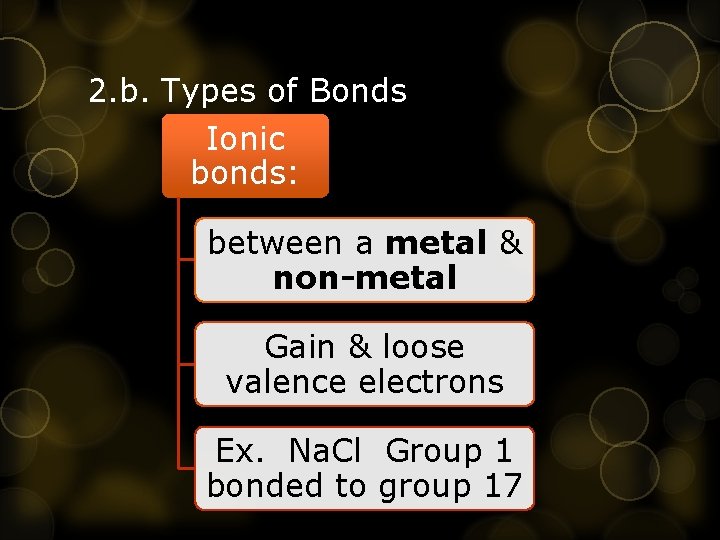
2. b. Types of Bonds Ionic bonds: between a metal & non-metal Gain & loose valence electrons Ex. Na. Cl Group 1 bonded to group 17

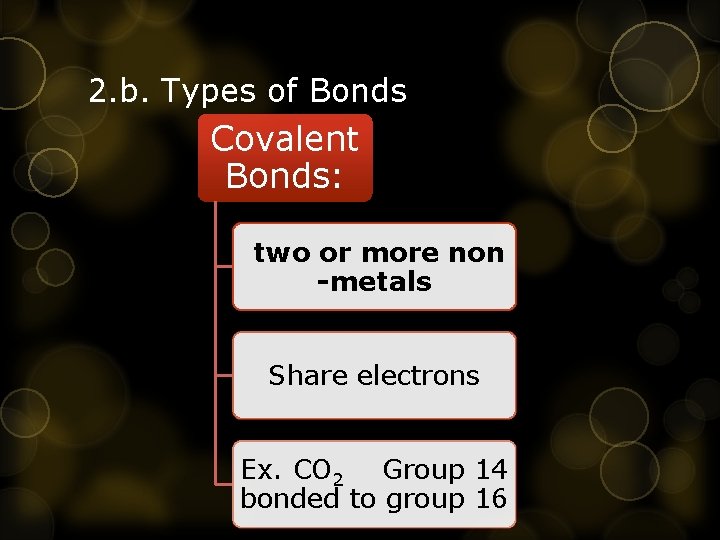
2. b. Types of Bonds Covalent Bonds: two or more non -metals Share electrons Ex. CO 2 Group 14 bonded to group 16

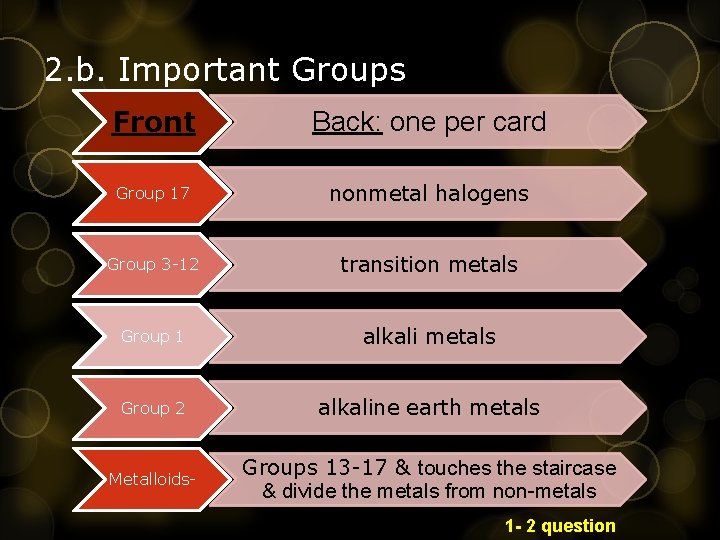
2. b. Important Groups Front Back: one per card Group 17 nonmetal halogens Group 3 -12 transition metals Group 1 alkali metals Group 2 alkaline earth metals Metalloids- Groups 13 -17 & touches the staircase & divide the metals from non-metals 1 - 2 question

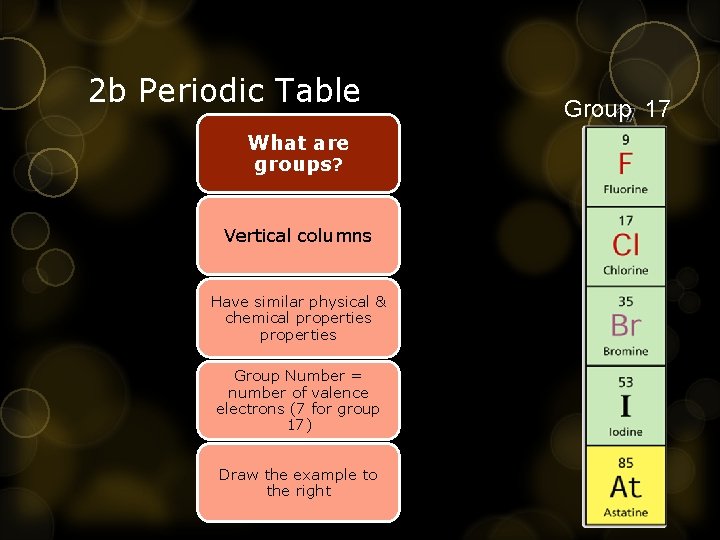
2 b Periodic Table What are groups? Vertical columns Have similar physical & chemical properties Group Number = number of valence electrons (7 for group 17) Draw the example to the right Group 17

Obj. 2. b. Acids • H+ (hydrogen ion) • Bond with nonmetals • Ex. HF weak acid • Ex. HI strong acid • p. H less than 7 • sour

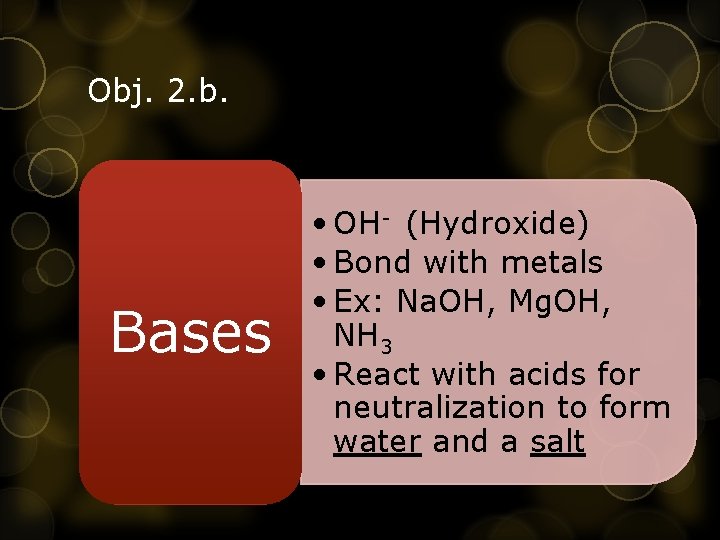
Obj. 2. b. Bases • OH- (Hydroxide) • Bond with metals • Ex: Na. OH, Mg. OH, NH 3 • React with acids for neutralization to form water and a salt

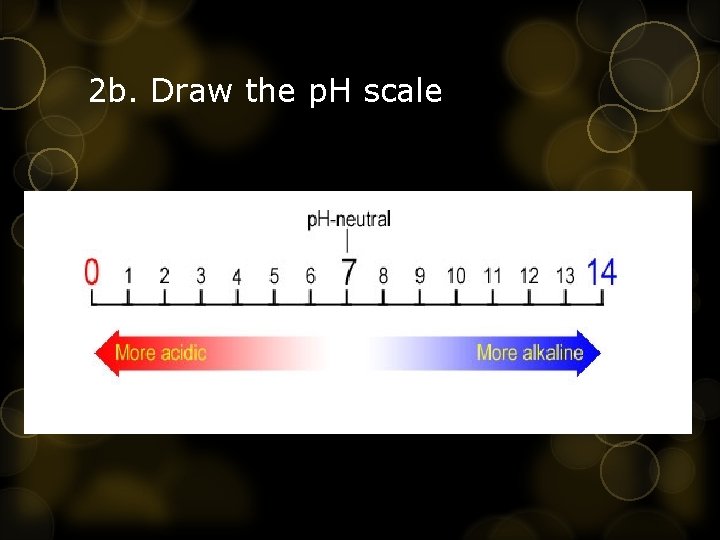
2 b. Draw the p. H scale

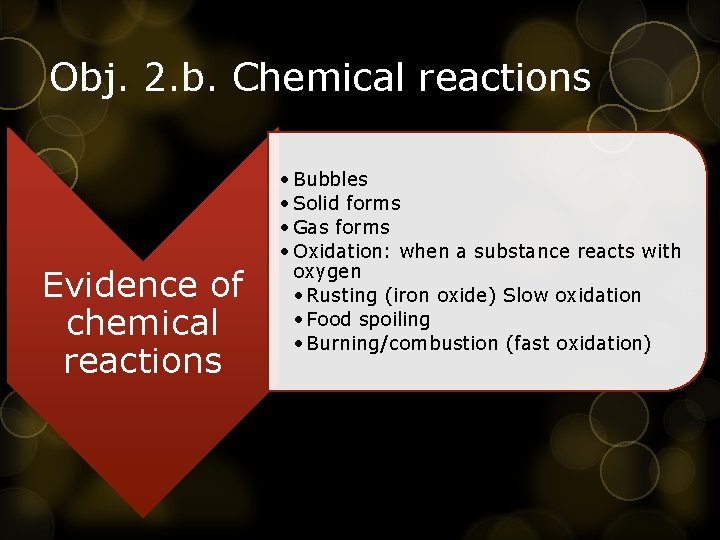
Obj. 2. b. Chemical reactions Evidence of chemical reactions • Bubbles • Solid forms • Gas forms • Oxidation: when a substance reacts with oxygen • Rusting (iron oxide) Slow oxidation • Food spoiling • Burning/combustion (fast oxidation)

Obj. 2. c. Motion What is the Speed formula What is Constant velocity What is acceleration? • s=d/t • no change in motion (balanced force) • ex. 50 mi/h for an hour • A change in motion due to an unbalanced force: speed up, slow down, change direction

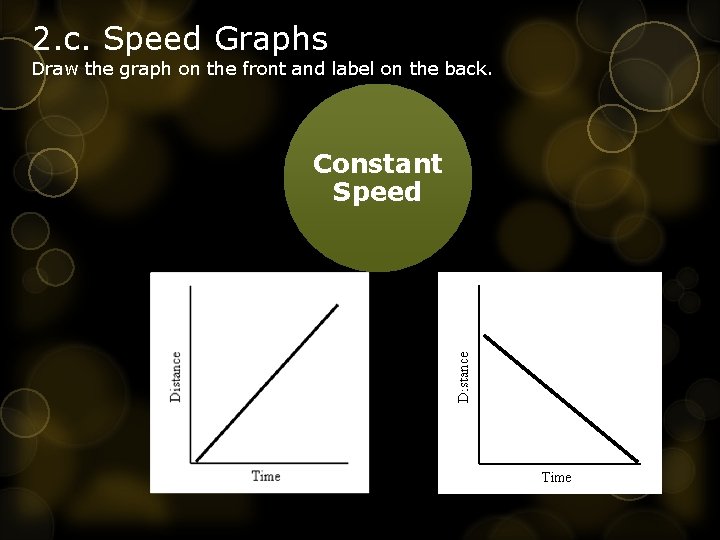
2. c. Speed Graphs Draw the graph on the front and label on the back. Constant Speed

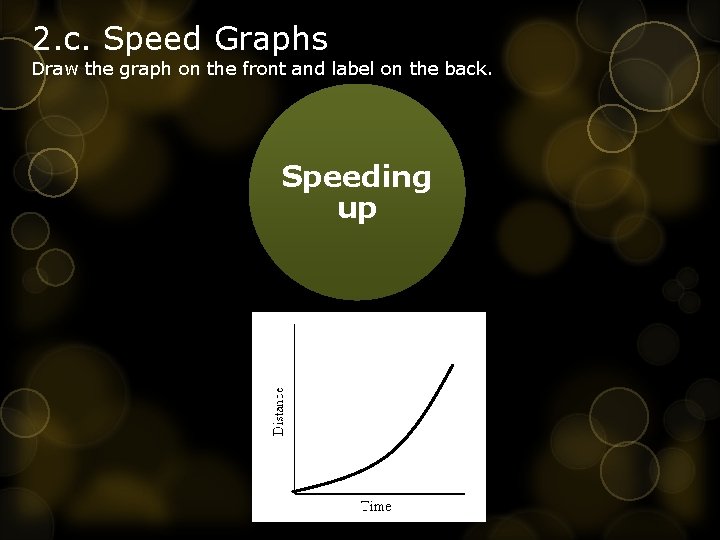
2. c. Speed Graphs Draw the graph on the front and label on the back. Speeding up

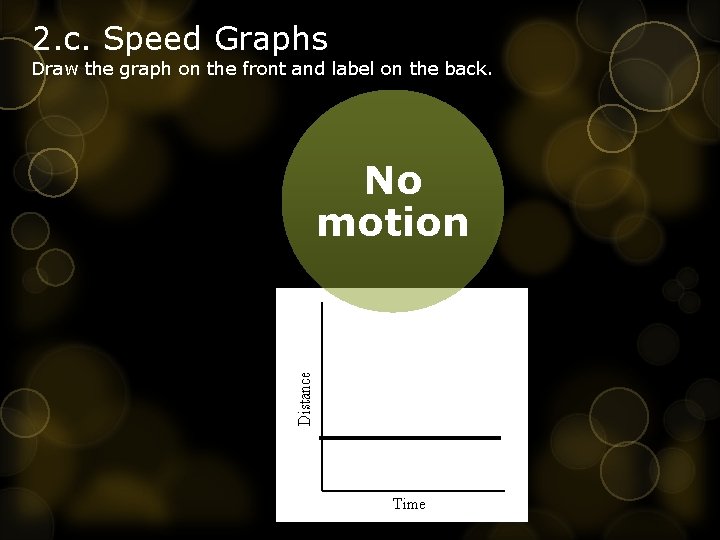
2. c. Speed Graphs Draw the graph on the front and label on the back. No motion

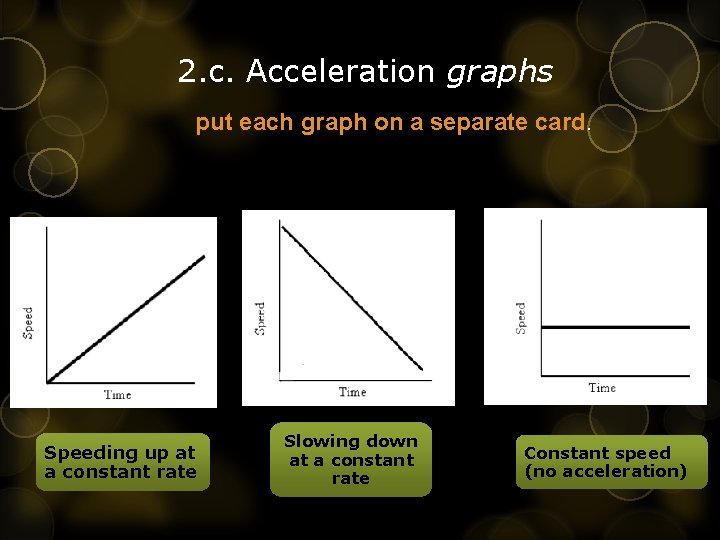
2. c. Acceleration graphs put each graph on a separate card. Label each: Speeding up at a constant rate Slowing down at a constant rate Constant speed (no acceleration)

Obj. 2. f. Newton’s Laws What is the 1 st law & give an example • (law of inertia): An object at rest will stay at rest. An object in motion will stay in motion until an unbalance force is applied. • Ex. Car stops, but your body moves forward • An object in moving in space will continue at a constant velocity (comet)

2. f. Newton’s Laws of Motion What is the 2 nd law & give an example • (F=ma) : an object will accelerate in the direction of the net force • Ex. Kick a ball upward and it moves upward • Objects with more mass require more force to move.

2. f. Newton’s Laws of Motion What is • (action/reaction): forces always act in the 3 rd equal but opposite law & pairs give an • Ex. Swimming, example birds flying, rockets

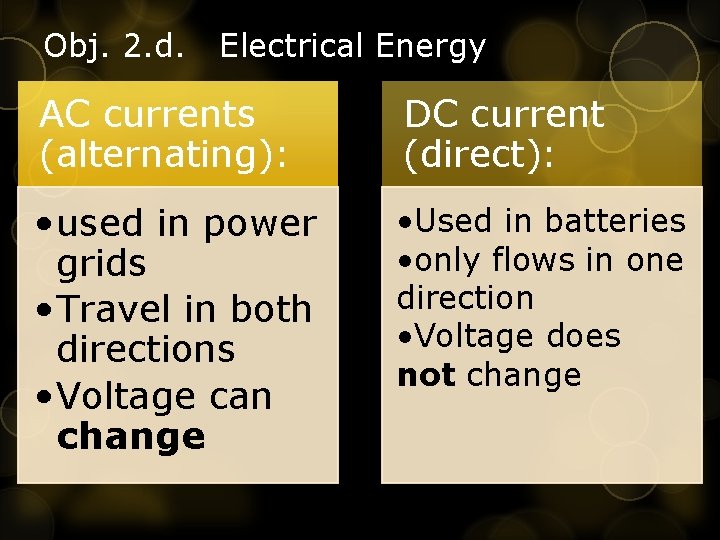
Obj. 2. d. Electrical Energy AC currents (alternating): DC current (direct): • used in power grids • Travel in both directions • Voltage can change • Used in batteries • only flows in one direction • Voltage does not change

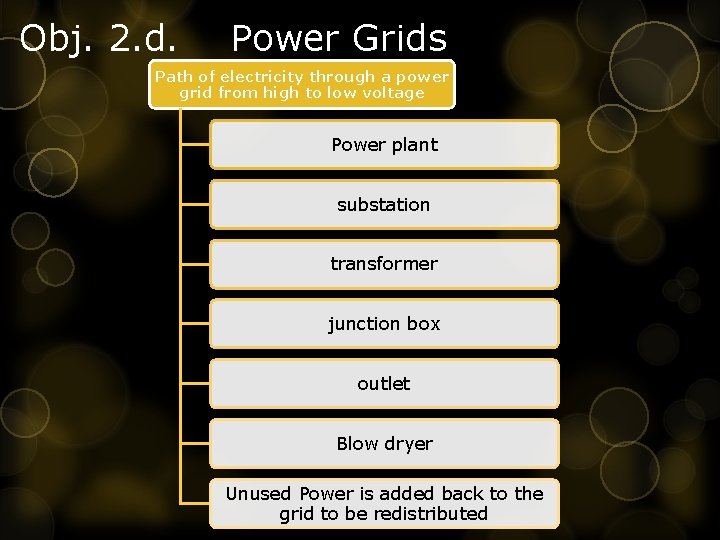
Obj. 2. d. Power Grids Path of electricity through a power grid from high to low voltage Power plant substation transformer junction box outlet Blow dryer Unused Power is added back to the grid to be redistributed

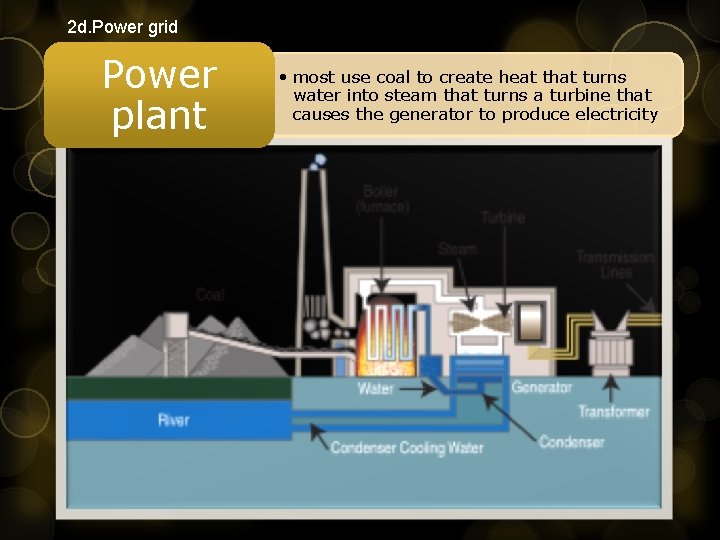
2 d. Power grid Power plant • most use coal to create heat that turns water into steam that turns a turbine that causes the generator to produce electricity

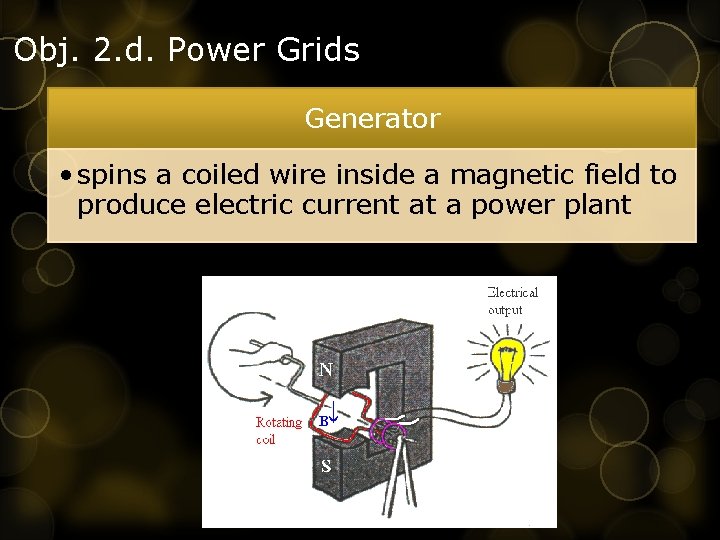
Obj. 2. d. Power Grids Generator • spins a coiled wire inside a magnetic field to produce electric current at a power plant

Obj. 2. d. Power Grids Transformer • Increases or decreases voltage in the power grid

Obj. 2. d. Power Grids What type of Renewable resources can be used to decrease the amount nonrenewable resources used to make electricity? • Wind • Solar- sun • Hydro - water • Geothermal – heat in Earth

Obj. 2. d. Power Grids What type of nonrenewable resources are used to make electricity? • Fossil Fuels • Coal • Natural gas • oil

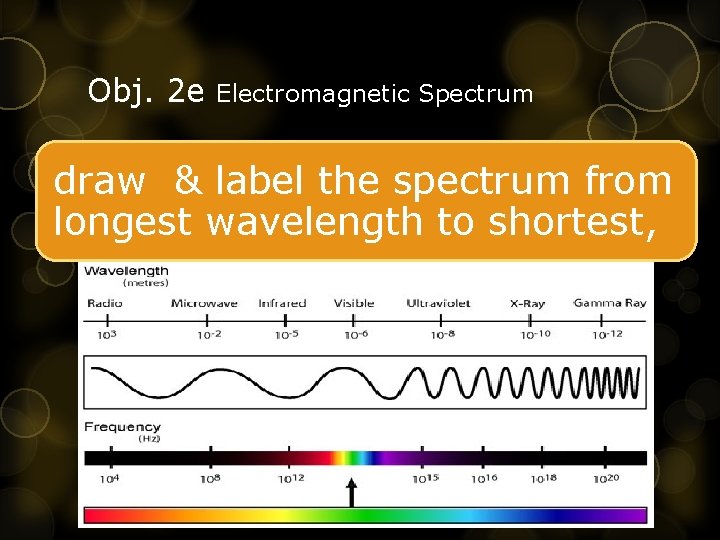
Obj. 2 e Electromagnetic Spectrum draw & label the spectrum from longest wavelength to shortest,

Obj. 2 e Electromagnetic Spectrum One card per radiation wave Infrared: • heat/thermal • lowest energy, longest wavelength Radio: communication • Used for communication Gamma Visible light • treat cancer • highest energy, shortest wavelength • roy. Gbiv- from (longest wavelength to shortest wavelength)

Obj. 2. e. Electromagnetic Spectrum Ultraviolet (UV) • blocked by ozone • damage skin cells, cause skin cancer • can be used to disinfect medical equipment, rid water of bacteria or microorganisms, etc.