Comparison of NNRTI vs PIr EFV vs LPVr



- Slides: 10

Comparison of NNRTI vs PI/r § EFV vs LPV/r vs EFV + LPV/r – A 5142 – Mexican Study § NVP vs ATV/r – ARTEN § EFV vs ATV/r – A 5202 § DOR vs DRV/r – DRIVE-FORWARD

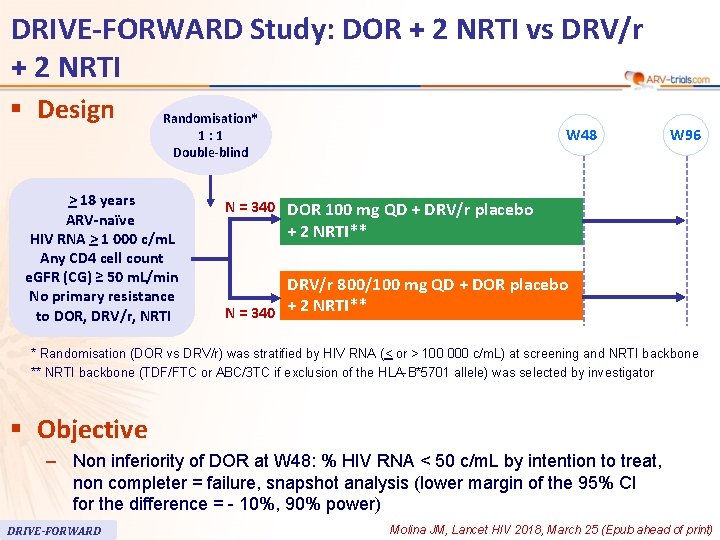
DRIVE-FORWARD Study: DOR + 2 NRTI vs DRV/r + 2 NRTI § Design Randomisation* 1: 1 Double-blind > 18 years ARV-naïve HIV RNA > 1 000 c/m. L Any CD 4 cell count e. GFR (CG) ≥ 50 m. L/min No primary resistance to DOR, DRV/r, NRTI W 48 W 96 N = 340 DOR 100 mg QD + DRV/r placebo + 2 NRTI** DRV/r 800/100 mg QD + DOR placebo N = 340 + 2 NRTI** * Randomisation (DOR vs DRV/r) was stratified by HIV RNA (< or > 100 000 c/m. L) at screening and NRTI backbone ** NRTI backbone (TDF/FTC or ABC/3 TC if exclusion of the HLA-B*5701 allele) was selected by investigator § Objective – Non inferiority of DOR at W 48: % HIV RNA < 50 c/m. L by intention to treat, non completer = failure, snapshot analysis (lower margin of the 95% CI for the difference = - 10%, 90% power) DRIVE-FORWARD Molina JM, Lancet HIV 2018, March 25 (Epub ahead of print)

DRIVE-FORWARD Study: DOR + 2 NRTI vs DRV/r + 2 NRTI Baseline characteristics and patient disposition DOR + 2 NRTI (N = 383) DRV/r + 2 NRTI (N = 383) Mean age, years 35 35 Female, % 17 15 AIDS, % 9 10 4. 4 22 19 433 412 11 17 87 / 13 88 / 13 56 (15%) 12 4 1 17 / 10 7/5 71 (19%) 14 12 0 19 / 13 4/9 HIV RNA (log 10 c/m. L), mean HIV RNA > 100 000 c/m. L, % CD 4 cell count (/mm 3), mean CD 4 < 200 per mm 3, % Selected NRTI: TDF/FTC / ABC/3 TC, % Discontinuation at W 48, N (%) Lack of efficacy, N Adverse event, N Death, N Lost to follow-up / Consent withdrawal, N Non-compliance / Other, N DRIVE-FORWARD Molina JM, Lancet HIV 2018, March 25 (Epub ahead of print)

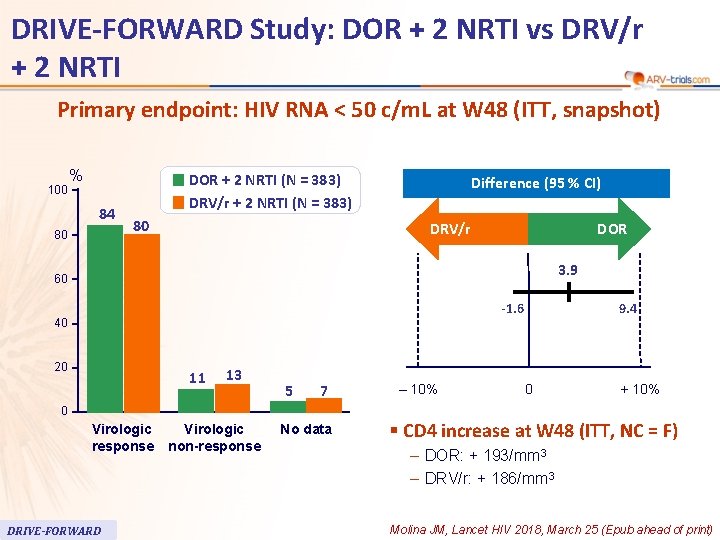
DRIVE-FORWARD Study: DOR + 2 NRTI vs DRV/r + 2 NRTI 58 Primary endpoint: HIV RNA < 50 c/m. L at W 48 (ITT, snapshot) 100 % 84 80 DOR + 2 NRTI (N = 383) DRV/r + 2 NRTI (N = 383) 80 Difference (95 % CI) DRV/r DOR 3. 9 60 -1. 6 40 20 11 13 5 7 0 Virologic response DRIVE-FORWARD Virologic non-response No data ‒ 10% 9. 4 0 + 10% § CD 4 increase at W 48 (ITT, NC = F) – DOR: + 193/mm 3 – DRV/r: + 186/mm 3 Molina JM, Lancet HIV 2018, March 25 (Epub ahead of print)

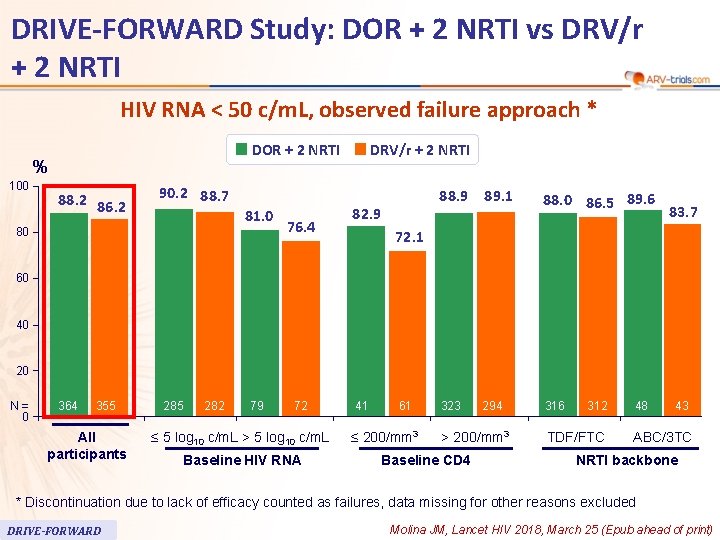
59 DRIVE-FORWARD Study: DOR + 2 NRTI vs DRV/r + 2 NRTI HIV RNA < 50 c/m. L, observed failure approach * DOR + 2 NRTI % 100 88. 2 86. 2 90. 2 88. 7 81. 0 80 76. 4 DRV/r + 2 NRTI 82. 9 88. 9 89. 1 88. 0 86. 5 89. 6 323 294 316 83. 7 72. 1 60 40 20 N= 0 364 71 355 All participants 285 282 79 72 ≤ 5 log 10 c/m. L > 5 log 10 c/m. L Baseline HIV RNA 41 61 ≤ 200/mm 3 > 200/mm 3 Baseline CD 4 312 TDF/FTC 48 43 ABC/3 TC NRTI backbone * Discontinuation due to lack of efficacy counted as failures, data missing for other reasons excluded DRIVE-FORWARD Molina JM, Lancet HIV 2018, March 25 (Epub ahead of print)

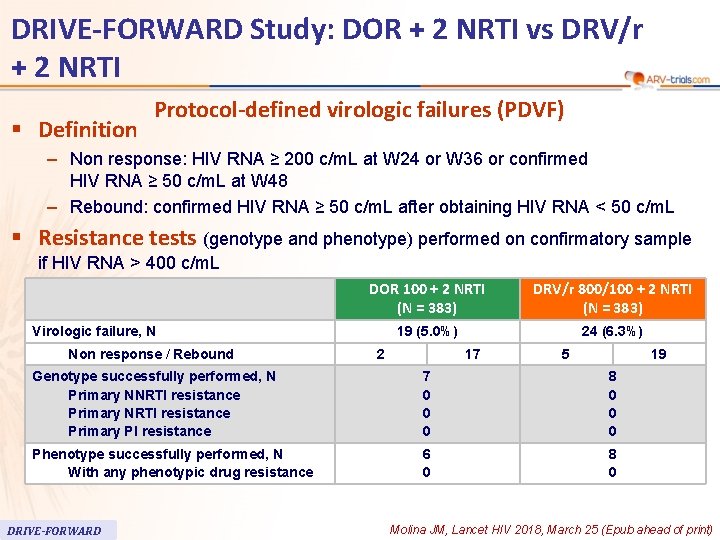
60 DRIVE-FORWARD Study: DOR + 2 NRTI vs DRV/r + 2 NRTI § Definition Protocol-defined virologic failures (PDVF) – Non response: HIV RNA ≥ 200 c/m. L at W 24 or W 36 or confirmed HIV RNA ≥ 50 c/m. L at W 48 – Rebound: confirmed HIV RNA ≥ 50 c/m. L after obtaining HIV RNA < 50 c/m. L § Resistance tests (genotype and phenotype) performed on confirmatory sample if HIV RNA > 400 c/m. L DOR 100 + 2 NRTI (N = 383) DRV/r 800/100 + 2 NRTI (N = 383) 19 (5. 0%) 24 (6. 3%) Virologic failure, N Non response / Rebound 2 17 5 19 Genotype successfully performed, N Primary NNRTI resistance Primary PI resistance 7 0 0 0 8 0 0 0 Phenotype successfully performed, N With any phenotypic drug resistance 6 0 8 0 DRIVE-FORWARD Molina JM, Lancet HIV 2018, March 25 (Epub ahead of print)

DRIVE-FORWARD Study: DOR + 2 NRTI vs DRV/r + 2 NRTI 61 Emergence of drug resistance in participants with discontinuations DOR 100 + 2 NRTI (N = 383 *) DRV/r 800/100 + 2 NRTI (N = 383) Discontinuation without PDVF, N (%) 40 (10. 4%) 53 (13. 9%) Genotype successfully performed, N Primary NNRTI resistance Primary PI resistance 2* 1 1 0 3 0 0 0 Phenotype successfully performed, N With any phenotypic drug resistance 2* 2 3 0 * 1 discontinuation for non-compliance at W 24, with emergence of resistance to DOR (V 106 I + H 221 Y ; > 90 fold increased IC 50) and FTC (M 184 V) ; 1 discontinuation for rash at W 2, with increased DOR IC 50 2. 8 fold WT (resistance cutoff = 2. 5 fold), but no genotypic resistance mutations DRIVE-FORWARD Molina JM, Lancet HIV 2018, March 25 (Epub ahead of print)

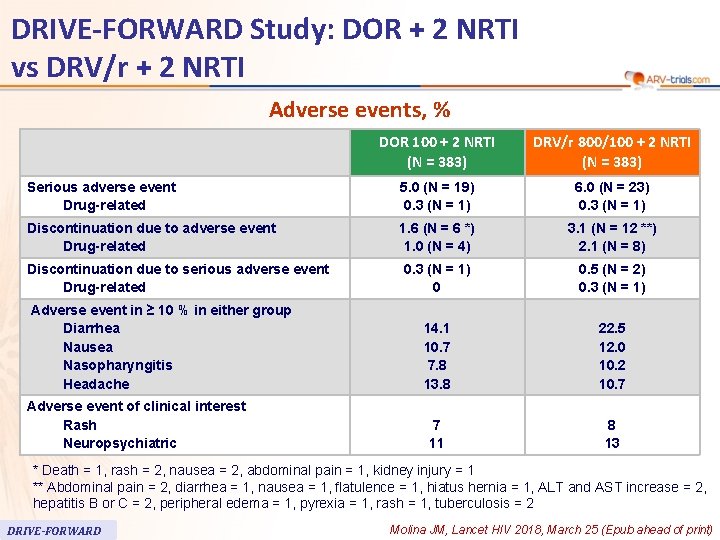
62 DRIVE-FORWARD Study: DOR + 2 NRTI vs DRV/r + 2 NRTI Adverse events, % DOR 100 + 2 NRTI (N = 383) DRV/r 800/100 + 2 NRTI (N = 383) Serious adverse event Drug-related 5. 0 (N = 19) 0. 3 (N = 1) 6. 0 (N = 23) 0. 3 (N = 1) Discontinuation due to adverse event Drug-related 1. 6 (N = 6 *) 1. 0 (N = 4) 3. 1 (N = 12 **) 2. 1 (N = 8) 0. 3 (N = 1) 0 0. 5 (N = 2) 0. 3 (N = 1) 14. 1 10. 7 7. 8 13. 8 22. 5 12. 0 10. 2 10. 7 7 11 8 13 Discontinuation due to serious adverse event Drug-related Adverse event in ≥ 10 % in either group Diarrhea Nausea Nasopharyngitis Headache Adverse event of clinical interest Rash Neuropsychiatric * Death = 1, rash = 2, nausea = 2, abdominal pain = 1, kidney injury = 1 ** Abdominal pain = 2, diarrhea = 1, nausea = 1, flatulence = 1, hiatus hernia = 1, ALT and AST increase = 2, hepatitis B or C = 2, peripheral edema = 1, pyrexia = 1, rash = 1, tuberculosis = 2 DRIVE-FORWARD Molina JM, Lancet HIV 2018, March 25 (Epub ahead of print)

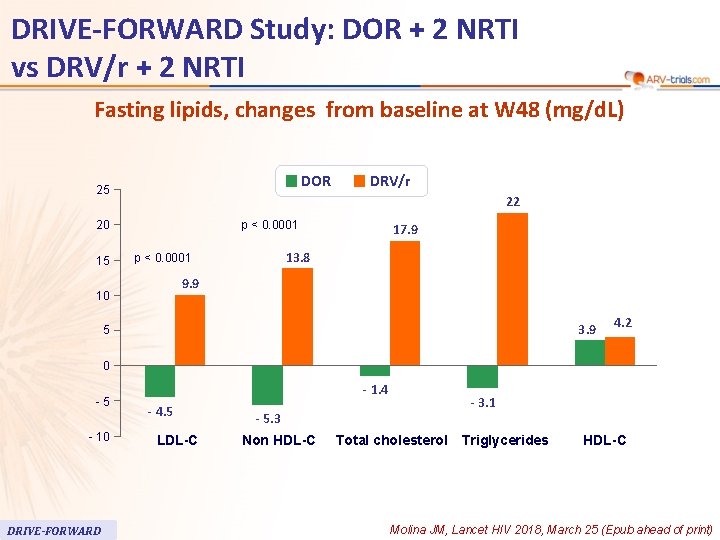
63 DRIVE-FORWARD Study: DOR + 2 NRTI vs DRV/r + 2 NRTI Fasting lipids, changes from baseline at W 48 (mg/d. L) DOR 25 22 p < 0. 0001 20 15 DRV/r 17. 9 13. 8 p < 0. 0001 9. 9 10 3. 9 5 4. 2 0 -5 - 10 DRIVE-FORWARD - 1. 4 - 4. 5 LDL-C - 3. 1 - 5. 3 Non HDL-C Total cholesterol Triglycerides HDL-C Molina JM, Lancet HIV 2018, March 25 (Epub ahead of print)

DRIVE-FORWARD Study: DOR + 2 NRTI vs DRV/r + 2 NRTI § Conclusion at Week 48 – DOR 100 mg QD, in combination with either TDF/FTC or ABC/3 TC • Achieved high virologic success at Week 48 • And was non-inferior to DRV/r + 2 NRTI regardless of baseline HIV RNA – Resistance mutations through 48 weeks • None were detected in protocol-defined virologic failures • Only 1/383 participants on DOR developed genotypic and phenotypic resistance to DOR + FTC/3 TC – Adverse events leading to discontinuation occurred with low frequency for both DOR and DRV/r • Low rate of discontinuation due to rash or neuropsychiatric adverse events – Lipid changes were less pronounced for DOR than for DRV/r – Once-daily DOR in combination with fixed-dose NRTIs represents an effective treatment option for HIV-1 -infected, treatment-naive patients DRIVE-FORWARD Molina JM, Lancet HIV 2018, March 25 (Epub ahead of print)