Comparison of NNRTI vs PIr EFV vs LPVr
- Slides: 5

Comparison of NNRTI vs PI/r § EFV vs LPV/r vs EFV + LPV/r – A 5142 – Mexican Study § NVP vs ATV/r – ARTEN § EFV vs ATV/r – A 5202

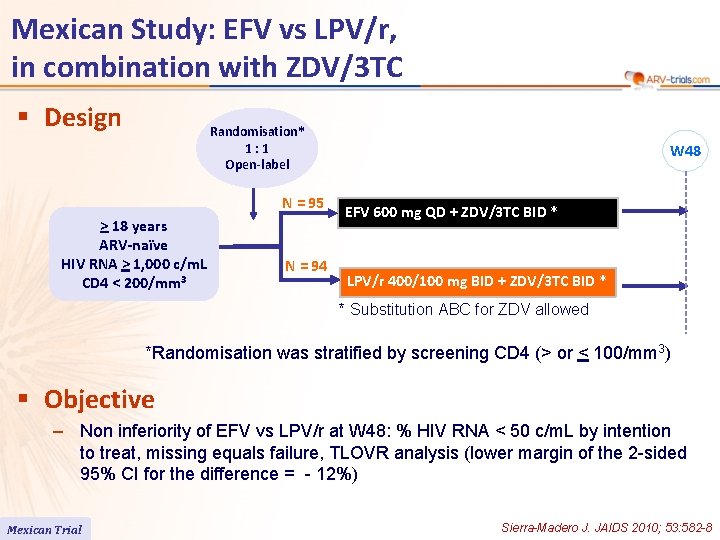
Mexican Study: EFV vs LPV/r, in combination with ZDV/3 TC § Design Randomisation* 1: 1 Open-label N = 95 > 18 years ARV-naïve HIV RNA > 1, 000 c/m. L CD 4 < 200/mm 3 N = 94 W 48 EFV 600 mg QD + ZDV/3 TC BID * LPV/r 400/100 mg BID + ZDV/3 TC BID * * Substitution ABC for ZDV allowed *Randomisation was stratified by screening CD 4 (> or < 100/mm 3) § Objective – Non inferiority of EFV vs LPV/r at W 48: % HIV RNA < 50 c/m. L by intention to treat, missing equals failure, TLOVR analysis (lower margin of the 2 -sided 95% CI for the difference = - 12%) Mexican Trial Sierra-Madero J. JAIDS 2010; 53: 582 -8

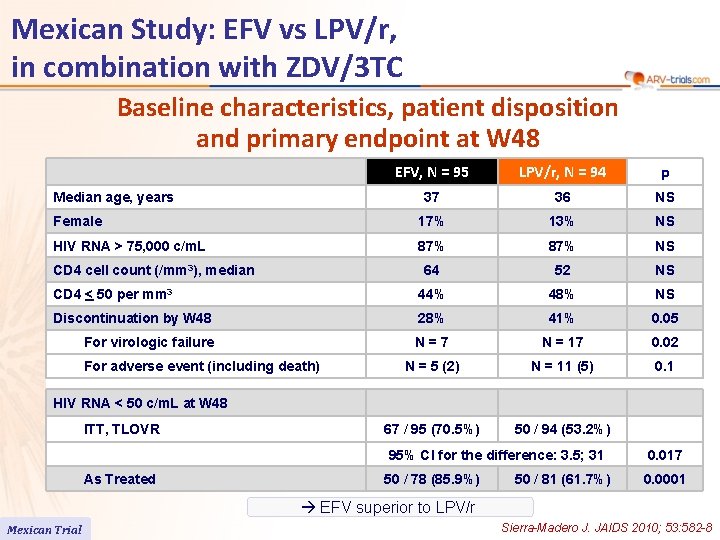
Mexican Study: EFV vs LPV/r, in combination with ZDV/3 TC Baseline characteristics, patient disposition and primary endpoint at W 48 EFV, N = 95 LPV/r, N = 94 p 37 36 NS Female 17% 13% NS HIV RNA > 75, 000 c/m. L 87% NS 64 52 NS CD 4 < 50 per mm 3 44% 48% NS Discontinuation by W 48 28% 41% 0. 05 For virologic failure N=7 N = 17 0. 02 N = 5 (2) N = 11 (5) 0. 1 67 / 95 (70. 5%) 50 / 94 (53. 2%) Median age, years CD 4 cell count (/mm 3), median For adverse event (including death) HIV RNA < 50 c/m. L at W 48 ITT, TLOVR 95% CI for the difference: 3. 5; 31 As Treated 50 / 78 (85. 9%) 50 / 81 (61. 7%) 0. 017 0. 0001 EFV superior to LPV/r Mexican Trial Sierra-Madero J. JAIDS 2010; 53: 582 -8

Mexican Study: EFV vs LPV/r, in combination with ZDV/3 TC § Secondary endpoints – HIV RNA < 50 c/m. L at W 48 according to baseline CD 4 • Baseline CD 4 < 100/mm 3 : EFV > LPV/r (p = 0. 03) • Baseline CD 4 > 100/mm 3 : virologic response to EFV and LPV/r not different (p = 0. 11) • Similar CD 4+ cell count increase in both groups – Incidence of grade 2 to 4 adverse events similar between groups: 68% • Significantly greater increase in triglyceride levels in LPV/r arm vs EFV (p < 0. 01) • Changes in total cholesterol, HDL, and LDL similar between groups – At virologic failure, only few patients were genotyped: • LPV/r, N = 5/17: no PI resistance, NRTI resistance in 1 • EFV, N = 3/7: NNRTI resistance in 3, NRTI resistance in 2 Mexican Trial Sierra-Madero J. JAIDS 2010; 53: 582 -8

Mexican Study: EFV vs LPV/r, in combination with ZDV/3 TC § Conclusion – In this very advanced HIV-infected antiretroviral-naïve population with a median CD 4 closed to 50/mm 3, EFV was virologically superior to LPV/r BID, when combined with ZDV/3 TC – EFV superiority was due to both a higher rate of virologic failure and of discontinuations due to adverse event in the LPV/r group – Limits • Single country study, limited sample size (underpowered) • LPV/r soft-gel capsules and high pill burden associated with low tolerability and poor adherence in advanced HIV disease • NRTI backbone: ZDV/3 TC Mexican Trial Sierra-Madero J. JAIDS 2010; 53: 582 -8