Comparision of Mutant Selection Window Hypothesis with Traditional



![Mutant Selection Window and Fluoroquinolone Pharmacokinetics: S. aureus Garenoxacin (BMS 284756) Ciprofloxacin [Drug] (mg/ml) Mutant Selection Window and Fluoroquinolone Pharmacokinetics: S. aureus Garenoxacin (BMS 284756) Ciprofloxacin [Drug] (mg/ml)](https://slidetodoc.com/presentation_image/2b5995f9a1cc4218bc2e2c1c16c7f7b0/image-18.jpg)




- Slides: 27

Comparision of Mutant Selection Window Hypothesis with Traditional Pharmacodynamics

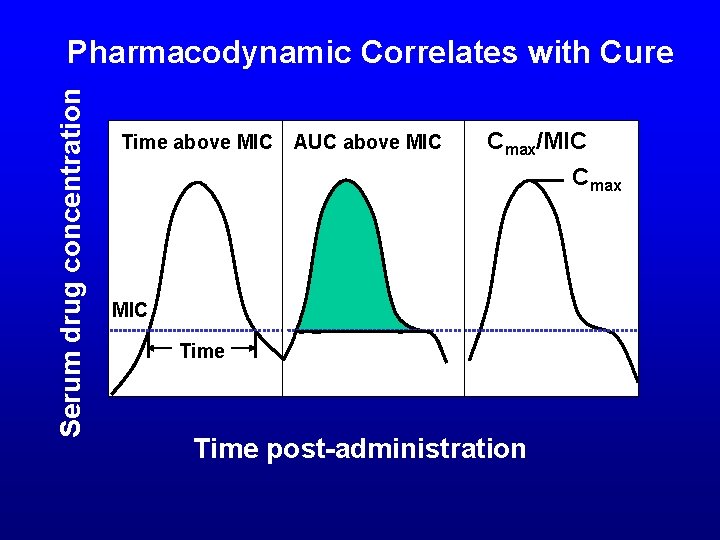
Serum drug concentration Pharmacodynamic Correlates with Cure Time above MIC AUC above MIC Cmax/MIC Cmax MIC Time post-administration

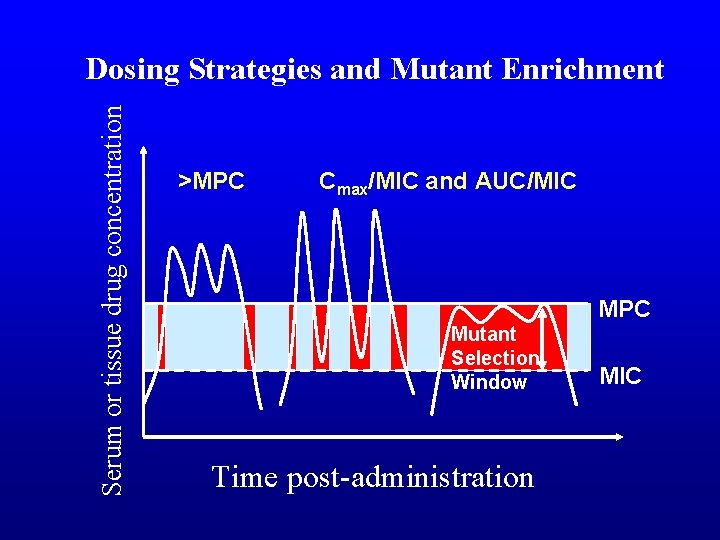
Serum or tissue drug concentration Dosing Strategies and Mutant Enrichment >MPC Cmax/MIC and AUC/MIC MPC Mutant Selection Window Time post-administration MIC

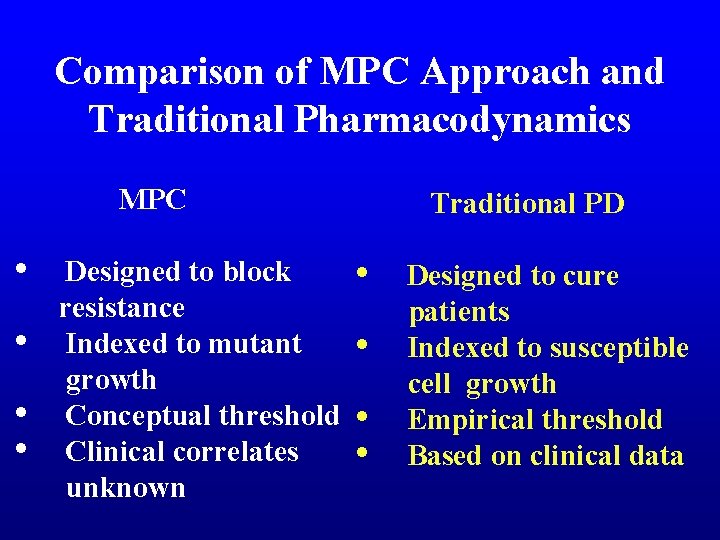
Comparison of MPC Approach and Traditional Pharmacodynamics MPC • • Designed to block resistance Indexed to mutant growth Conceptual threshold Clinical correlates unknown Traditional PD • • Designed to cure patients Indexed to susceptible cell growth Empirical threshold Based on clinical data

Applications to Streptococcus neumoniae

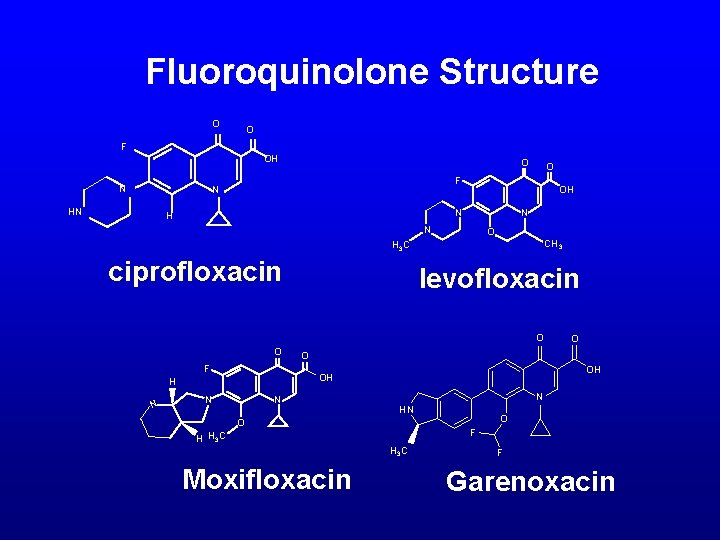
Fluoroquinolone Structure O O F OH N O N HN O F OH N N O CH 3 H 3 C ciprofloxacin levofloxacin O O F O OH OH H N N O N HN O F H H 3 C Moxifloxacin O F Garenoxacin N

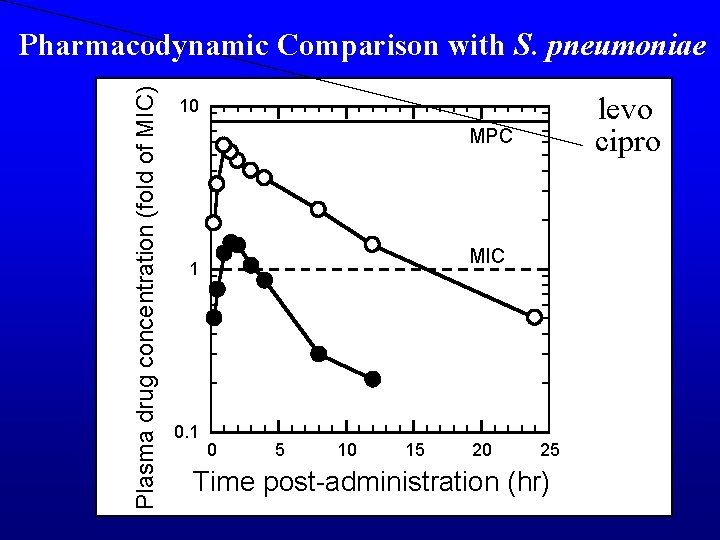
Plasma drug concentration (fold of MIC) Pharmacodynamic Comparison with S. pneumoniae levo cipro 10 MPC MIC 1 0 5 10 15 20 25 Time post-administration (hr)

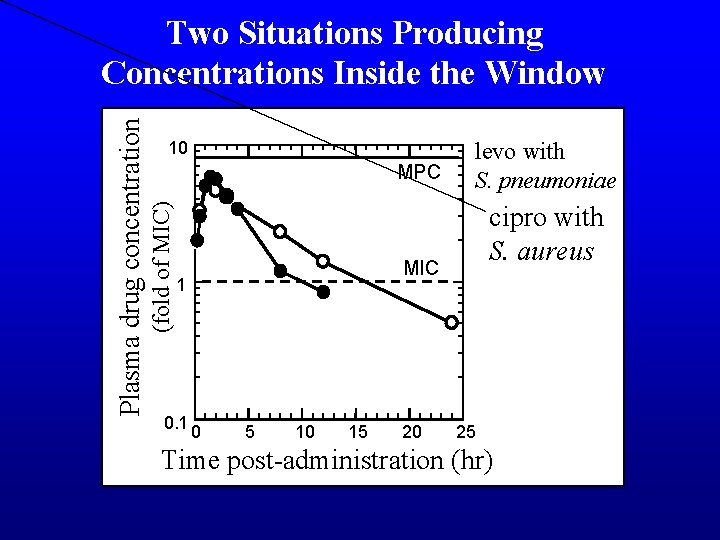
10 MPC (fold of MIC) Plasma drug concentration Two Situations Producing Concentrations Inside the Window cipro with S. aureus MIC 1 0. 1 levo with S. pneumoniae 0 5 10 15 20 25 Time post-administration (hr)

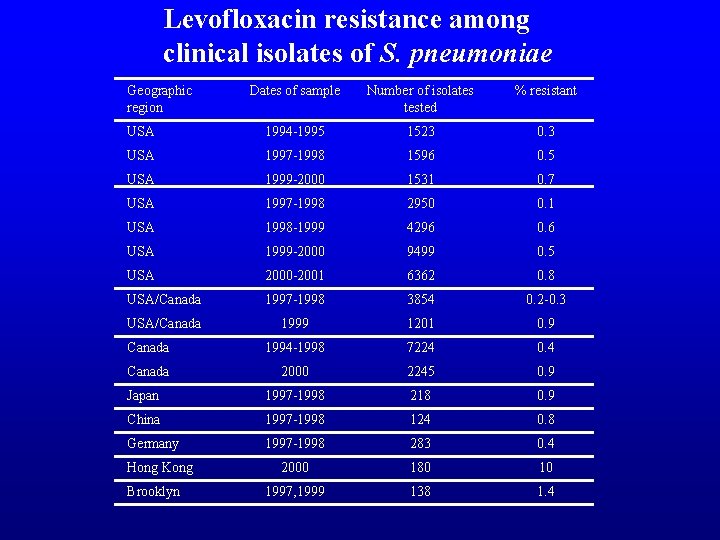
Levofloxacin resistance among clinical isolates of S. pneumoniae Geographic region Dates of sample Number of isolates tested % resistant USA 1994 -1995 1523 0. 3 USA 1997 -1998 1596 0. 5 USA 1999 -2000 1531 0. 7 USA 1997 -1998 2950 0. 1 USA 1998 -1999 4296 0. 6 USA 1999 -2000 9499 0. 5 USA 2000 -2001 6362 0. 8 USA/Canada 1997 -1998 3854 0. 2 -0. 3 USA/Canada 1999 1201 0. 9 Canada 1994 -1998 7224 0. 4 Canada 2000 2245 0. 9 Japan 1997 -1998 218 0. 9 China 1997 -1998 124 0. 8 Germany 1997 -1998 283 0. 4 2000 180 10 1997, 1999 138 1. 4 Hong Kong Brooklyn

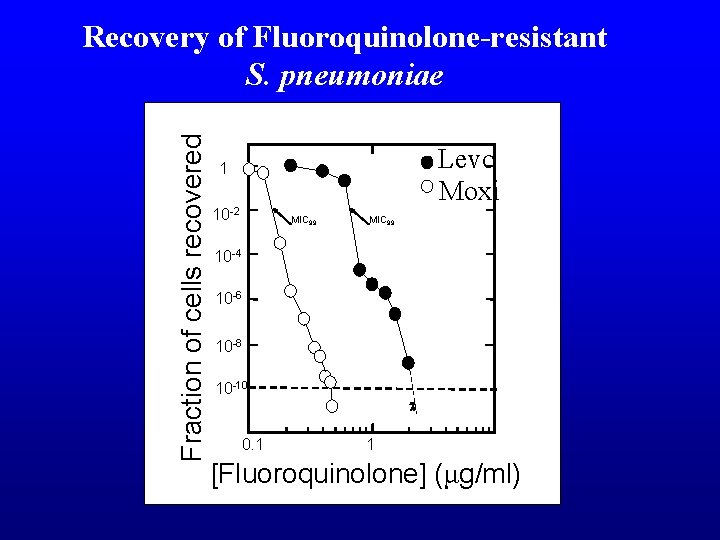
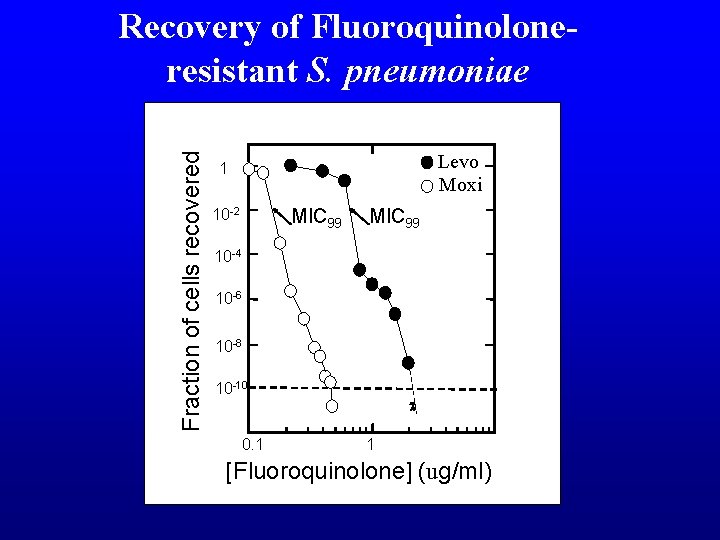
Fraction of cells recovered Recovery of Fluoroquinolone-resistant S. pneumoniae Levo Moxi 1 10 -2 MIC 99 10 -4 10 -6 10 -8 10 -10 0. 1 1 [Fluoroquinolone] (μg/ml)

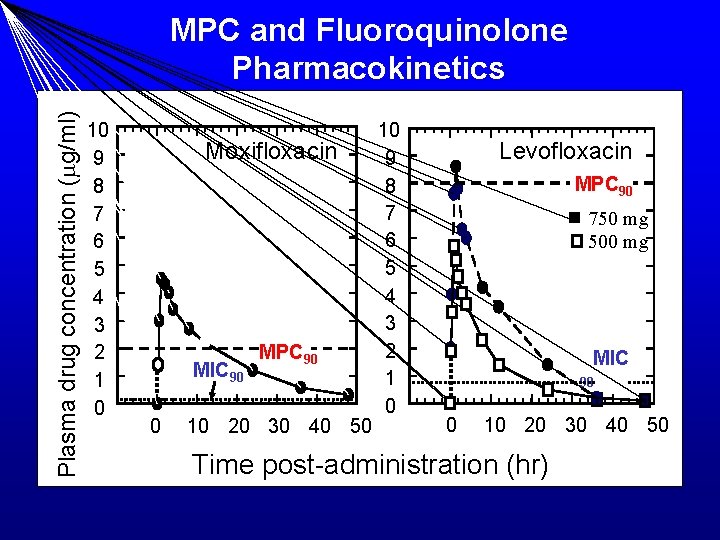
Plasma drug concentration (mg/ml) MPC and Fluoroquinolone Pharmacokinetics 10 9 8 7 6 5 4 3 2 1 0 Moxifloxacin MIC 90 0 MPC 90 10 20 30 40 50 10 9 8 7 6 5 4 3 2 1 0 Levofloxacin MPC 90 750 mg 500 mg MIC 90 0 10 20 30 40 50 Time post-administration (hr)

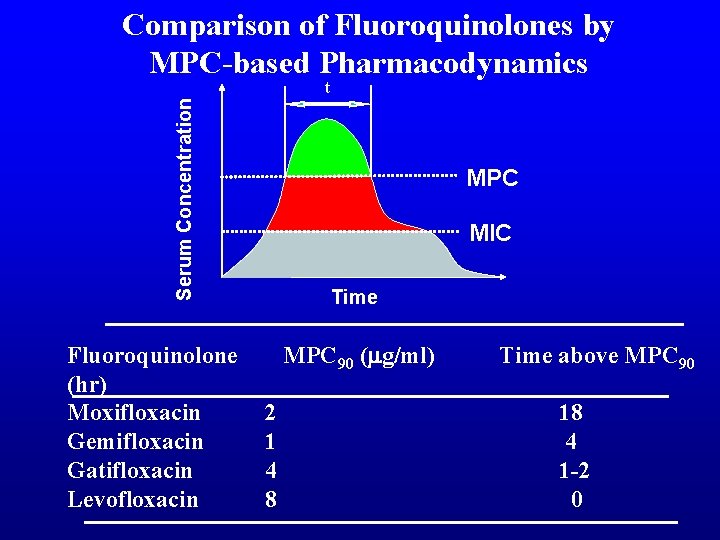
Comparison of Fluoroquinolones by MPC-based Pharmacodynamics Serum Concentration t Fluoroquinolone (hr) Moxifloxacin Gemifloxacin Gatifloxacin Levofloxacin MPC MIC Time MPC 90 (mg/ml) 2 1 4 8 Time above MPC 90 18 4 1 -2 0

Fraction of cells recovered Recovery of Fluoroquinoloneresistant S. pneumoniae Levo Moxi 1 MIC 99 10 -2 MIC 99 10 -4 10 -6 10 -8 10 -10 0. 1 1 [Fluoroquinolone] (ug/ml)

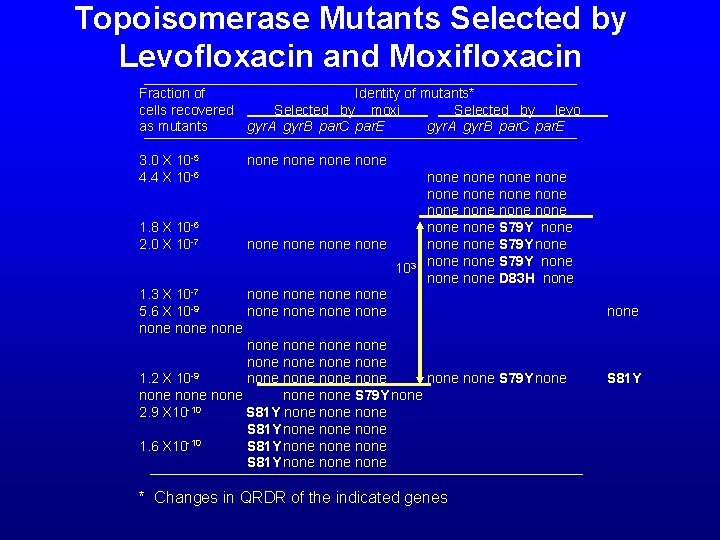
Topoisomerase Mutants Selected by Levofloxacin and Moxifloxacin Fraction of Identity of mutants* cells recovered Selected by moxi Selected by levo as mutants gyr. A gyr. B par. C par. E 3. 0 X 10 -5 4. 4 X 10 -6 1. 8 X 10 -6 2. 0 X 10 -7 none none 103 1. 3 X 10 -7 none -9 5. 6 X 10 none none none -9 1. 2 X 10 none none -10 2. 9 X 10 S 81 Y none -10 1. 6 X 10 S 81 Y none none none none none none S 79 Y none D 83 H none none S 79 Y none none * Changes in QRDR of the indicated genes none S 81 Y

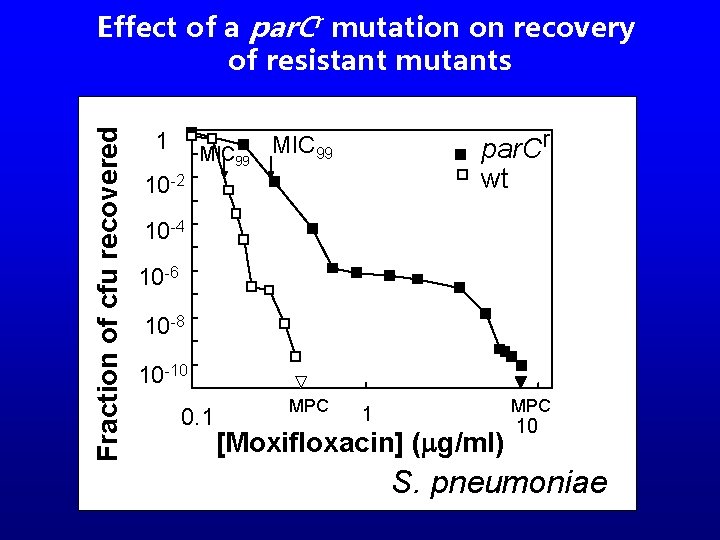
Fraction of cfu recovered Effect of a par. Cr mutation on recovery of resistant mutants 1 MIC 99 par. Cr wt MIC 99 10 -2 10 -4 10 -6 10 -8 10 -10 0. 1 MPC 1 [Moxifloxacin] (mg/ml) 10 S. pneumoniae

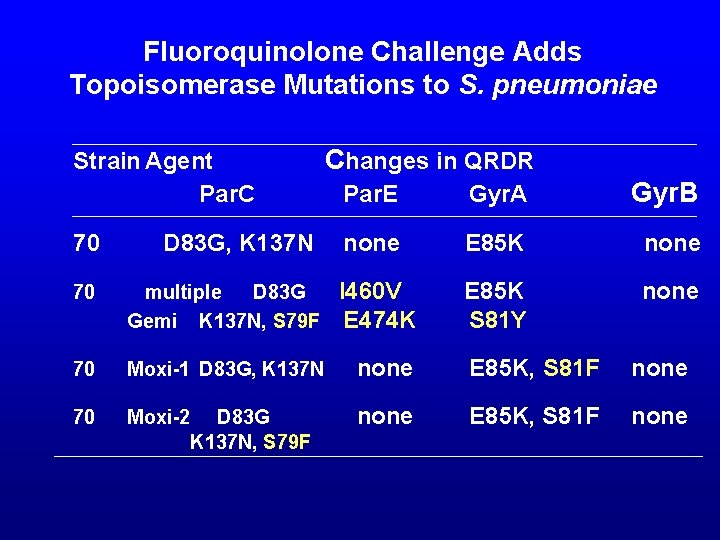
Fluoroquinolone Challenge Adds Topoisomerase Mutations to S. pneumoniae Strain Agent Par. C Changes in QRDR Par. E Gyr. A Gyr. B D 83 G, K 137 N none E 85 K none 70 70 multiple D 83 G I 460 V Gemi K 137 N, S 79 F E 474 K 70 Moxi-1 D 83 G, K 137 N none 70 Moxi-2 D 83 G K 137 N, S 79 F none S 81 Y E 85 K, S 81 F none

Applications to Staphylococcus aureus
![Mutant Selection Window and Fluoroquinolone Pharmacokinetics S aureus Garenoxacin BMS 284756 Ciprofloxacin Drug mgml Mutant Selection Window and Fluoroquinolone Pharmacokinetics: S. aureus Garenoxacin (BMS 284756) Ciprofloxacin [Drug] (mg/ml)](https://slidetodoc.com/presentation_image/2b5995f9a1cc4218bc2e2c1c16c7f7b0/image-18.jpg)
Mutant Selection Window and Fluoroquinolone Pharmacokinetics: S. aureus Garenoxacin (BMS 284756) Ciprofloxacin [Drug] (mg/ml) 10 7 MPC 90 30 6 cip. S 5 4 3 1 2 1 MIC 90 MPC 90 0. 03 0. 2 0 2 4 6 8 MIC 90 0 5 10 15 20 25 MPC 90 cip. R 16 14 12 10 8 6 4 2 0 MIC 90 0 Time post-administration (hr) 5 10 15 20 25

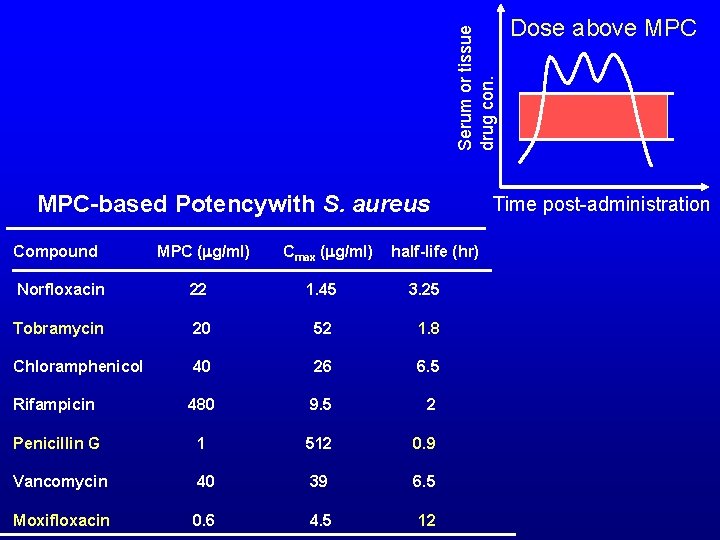
Serum or tissue drug con. MPC-based Potencywith S. aureus Compound MPC (mg/ml) Norfloxacin 22 Cmax (mg/ml) half-life (hr) 1. 45 3. 25 20 52 1. 8 Chloramphenicol 40 26 6. 5 Rifampicin 480 9. 5 2 Penicillin G 1 512 0. 9 Tobramycin Vancomycin 40 39 6. 5 Moxifloxacin 0. 6 4. 5 12 Dose above MPC Time post-administration

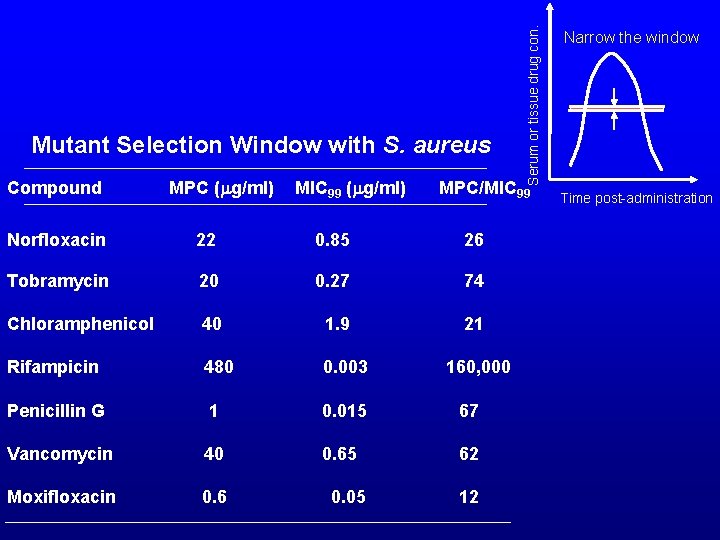
Compound MPC (mg/ml) MIC 99 (mg/ml) MPC/MIC 99 Norfloxacin 22 0. 85 26 Tobramycin 20 0. 27 74 40 1. 9 21 Chloramphenicol Serum or tissue drug con. Mutant Selection Window with S. aureus Rifampicin 480 0. 003 Penicillin G 1 0. 015 67 Vancomycin 40 0. 65 62 Moxifloxacin 0. 6 0. 05 160, 000 12 Narrow the window Time post-administration

Is the Mutant Selection Window Restricted to Fluoroquinolones?

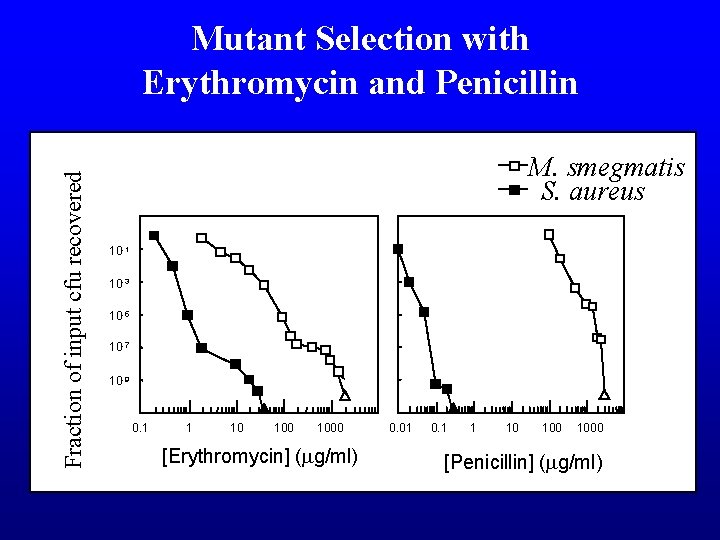
Fraction of input cfu recovered Mutant Selection with Erythromycin and Penicillin M. smegmatis S. aureus 10 -1 10 -3 10 -5 10 -7 10 -9 0. 1 1 10 1000 [Erythromycin] (mg/ml) 0. 01 0. 1 1 10 1000 [Penicillin] (mg/ml)

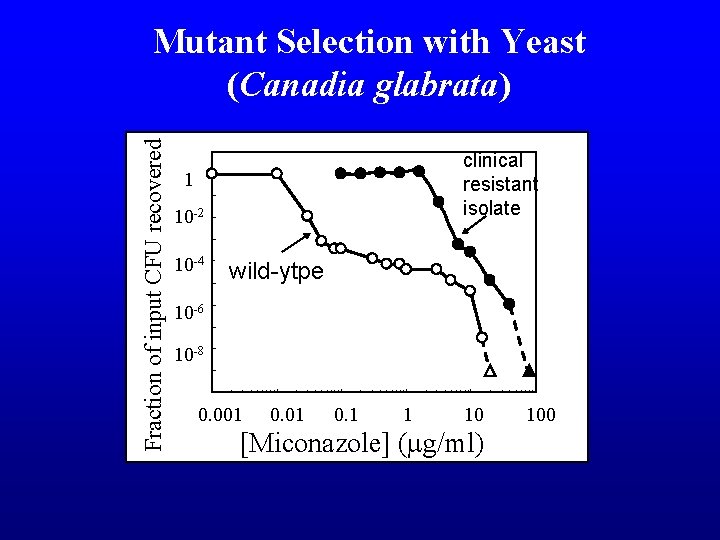
Fraction of input CFU recovered Mutant Selection with Yeast (Canadia glabrata) clinical resistant isolate 1 10 -2 10 -4 wild-ytpe 10 -6 10 -8 0. 001 0. 1 1 10 [Miconazole] (mg/ml) 100

Why is the Selection Window Hypothesis Useful?

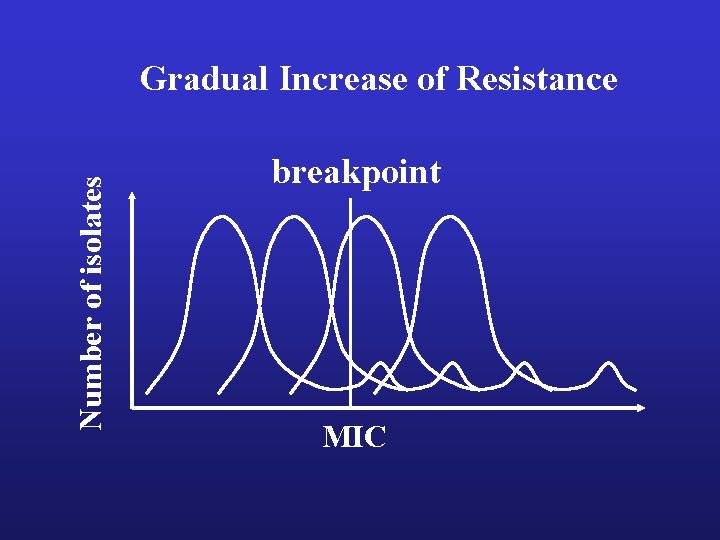
Number of isolates Gradual Increase of Resistance breakpoint MIC

Problems for implementing direct attack of mutants • No animal or clinical studies done • Currently requires altruism (dosing higher than needed for cure) • With S. pneumoniae time is running out (cross-resistance)

Literature Cited