Comparing two times weekly to three times weekly











- Slides: 23

Comparing two times weekly to three times weekly prophylaxis with Trimethoprim/Sulfamethoxazole for Pneumocystic jiroveci (carnii) pneumonia in pediatric oncology patients Elhaam Mesghali, Pharm. D. PGY-2 Pediatric Pharmacy Resident Loma Linda University Children’s Hospital | School of Pharmacy IRB Approved

Disclosure • Elhaam Mesghali • Potential Conflicts of Interest: None • Sponsorship: None • Proprietary information or results of ongoing research may be subject to different interpretations • Speaker’s presentation is educational in nature and indicates agreement to abide by non-commercialism guidelines provided 2

Learning Objectives » Identify the Pneumocystis jiroveci Pneumonia (PJP) prophylaxis regimen specified by National Comprehensive Cancer Network (NCCN) for pediatric oncology patients » Determine the difference in efficacy in patients who received three times weekly versus two times weekly Trimethoprim/Sulfamethoxazole as a prophylactic regimen. 3

Background » Pneumocystis jiroveci pneumonia (PJP) ~ Opportunistic infection ~ Requires prophylaxis in immunocompromised patients » Prophylaxis options ~ TMP/SMX 5 mg/kg/day on 3 consecutive days or on alternate days ~ Pentamidine 300 mg inhaled monthly ~ Dapsone 4 mg/kg (max 400 mg) weekly or 2 mg/kg daily (max 100 mg) ~ Atovaquone 30 mg/kg (max 1500 mg) daily American Academy of Pediatrics. 2015 “Prevention and Treatment of Cancer-Related Infections. ” National Comprehensive Cancer Network, 1 Dec. 2017, 4

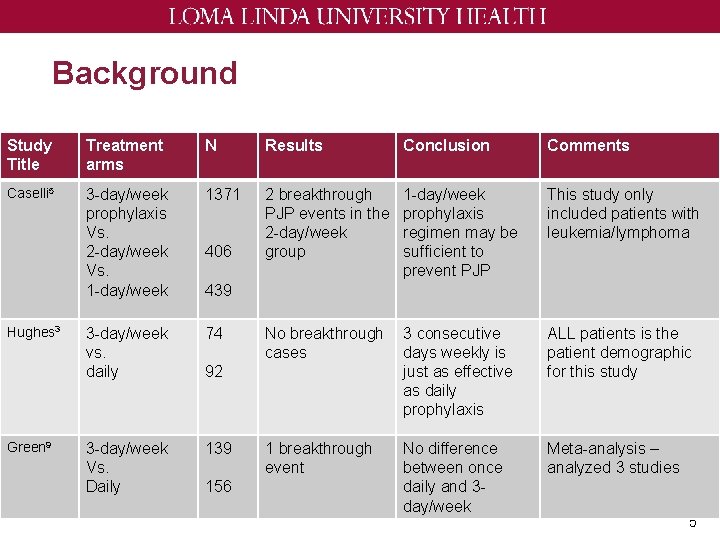
Background Study Title Treatment arms N Results Conclusion Comments Caselli 5 3 -day/week prophylaxis Vs. 2 -day/week Vs. 1 -day/week 1371 2 breakthrough PJP events in the 2 -day/week group 1 -day/week prophylaxis regimen may be sufficient to prevent PJP This study only included patients with leukemia/lymphoma 3 -day/week vs. daily 74 No breakthrough cases 3 consecutive days weekly is just as effective as daily prophylaxis ALL patients is the patient demographic for this study 3 -day/week Vs. Daily 139 1 breakthrough event No difference between once daily and 3 day/week Meta-analysis – analyzed 3 studies Hughes 3 Green 9 406 439 92 156 5

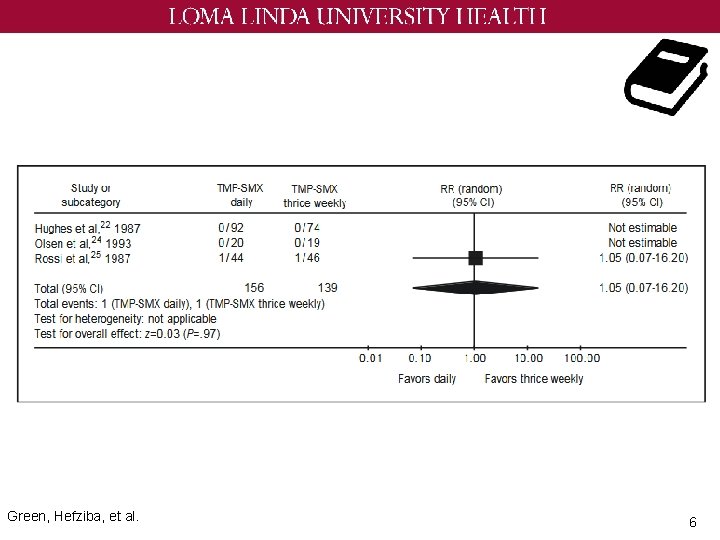
Green, Hefziba, et al. 6

Study Objectives » Purpose: Describe the efficacy of 2 and 3 consecutive prophylaxis days per week of TMP/SMX for prevention of PJP in pediatric patients with leukemia, lymphoma and solids tumors. 7

Study Design Retrospective chart review of patients from February 1 st 2013 to July 31 st 2017 who were administered either three times weekly or two times weekly Sulfamethoxazole/trimethoprim at LLUCH. Inclusion - Aged 21 years or younger - Oncology patients (hematologic and solid tumors) - Received either Sat/Sun prophylaxis regimen or three times weekly 8

Study Design Retrospective chart review of patients from February 1 st 2013 to July 31 st 2017 who were administered either three times weekly or two times weekly Sulfamethoxazole/trimethoprim at LLUCH. Exclusion Start time of TMP/SMX unclear Switch to alternative prophylaxis occurred within one month of TMP/SMX initiation 9

PJP definition Diagnosis of PJP ~ Stain positive ~ Radiological findings (CT, CXR) may suggest PJP Suspected PJP ~ Determined by exclusion 10

Results 11

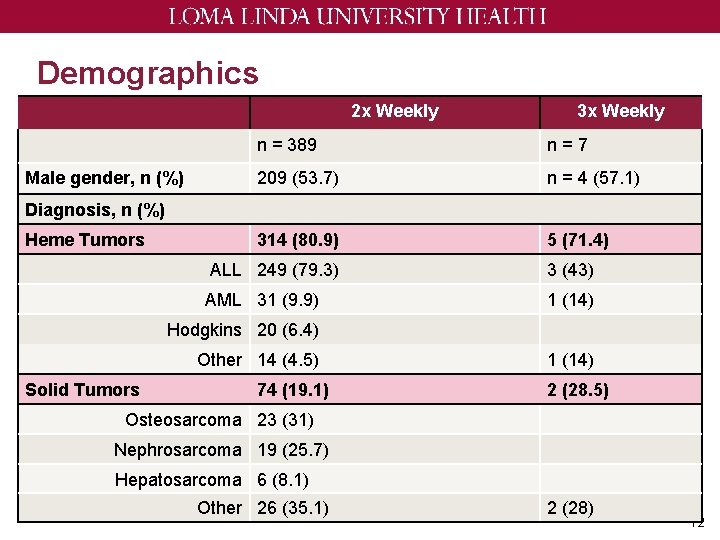
Demographics 2 x Weekly Male gender, n (%) 3 x Weekly n = 389 n=7 209 (53. 7) n = 4 (57. 1) 314 (80. 9) 5 (71. 4) Diagnosis, n (%) Heme Tumors ALL 249 (79. 3) 3 (43) AML 31 (9. 9) 1 (14) Hodgkins 20 (6. 4) Other 14 (4. 5) Solid Tumors 74 (19. 1) 1 (14) 2 (28. 5) Osteosarcoma 23 (31) Nephrosarcoma 19 (25. 7) Hepatosarcoma 6 (8. 1) Other 26 (35. 1) 2 (28) 12

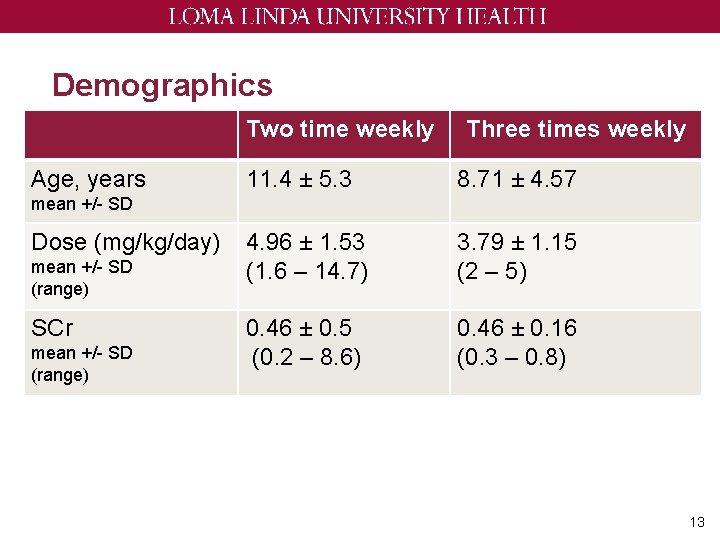
Demographics Two time weekly Age, years Three times weekly 11. 4 ± 5. 3 8. 71 ± 4. 57 4. 96 ± 1. 53 (1. 6 – 14. 7) 3. 79 ± 1. 15 (2 – 5) 0. 46 ± 0. 5 (0. 2 – 8. 6) 0. 46 ± 0. 16 (0. 3 – 0. 8) mean +/- SD Dose (mg/kg/day) mean +/- SD (range) SCr mean +/- SD (range) 13

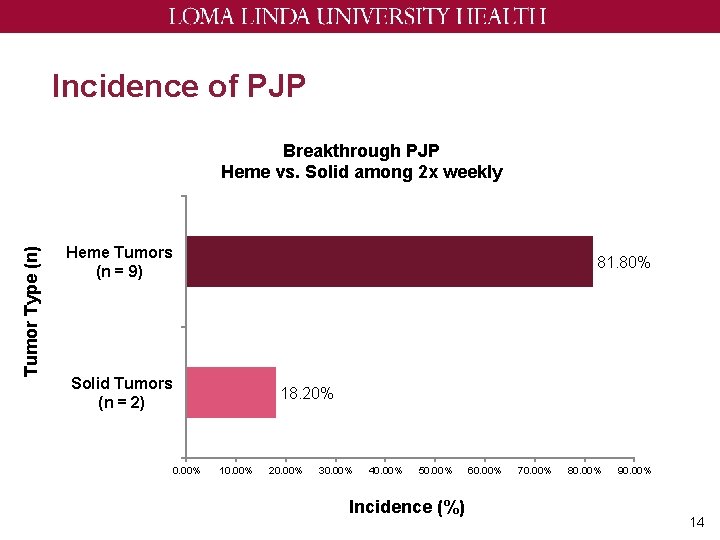
Incidence of PJP Tumor Type (n) Breakthrough PJP Heme vs. Solid among 2 x weekly Heme Tumors (n = 9) 81. 80% Solid Tumors (n = 2) 0. 00% 18. 20% 10. 00% 20. 00% 30. 00% 40. 00% 50. 00% Incidence (%) 60. 00% 70. 00% 80. 00% 90. 00% 14

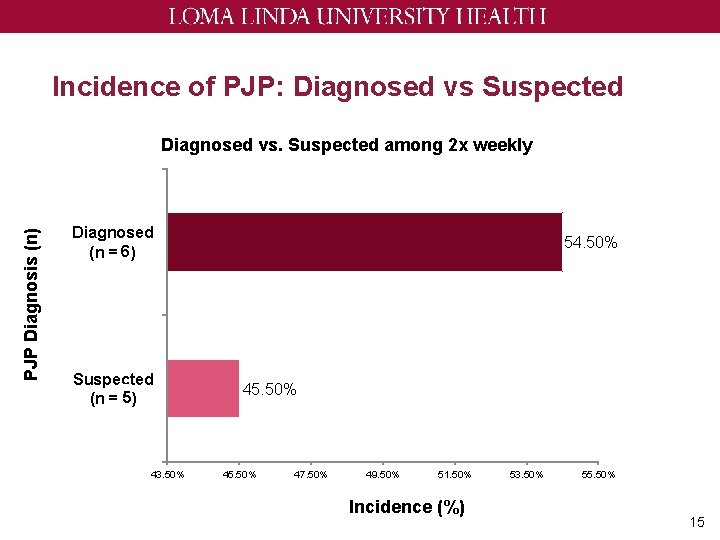
Incidence of PJP: Diagnosed vs Suspected PJP Diagnosis (n) Diagnosed vs. Suspected among 2 x weekly Diagnosed (n = 65) Suspected (n = 5 6) 43. 50% 54. 50% 45. 50% 47. 50% 49. 50% 51. 50% Incidence (%) 53. 50% 55. 50% 15

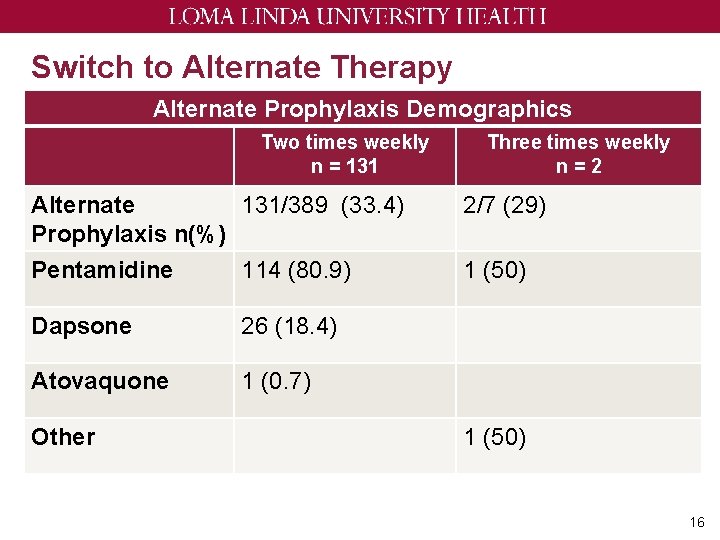
Switch to Alternate Therapy Alternate Prophylaxis Demographics Two times weekly n = 131 Three times weekly n=2 Alternate 131/389 (33. 4) Prophylaxis n(%) 2/7 (29) Pentamidine 114 (80. 9) 1 (50) Dapsone 26 (18. 4) Atovaquone 1 (0. 7) Other 1 (50) 16

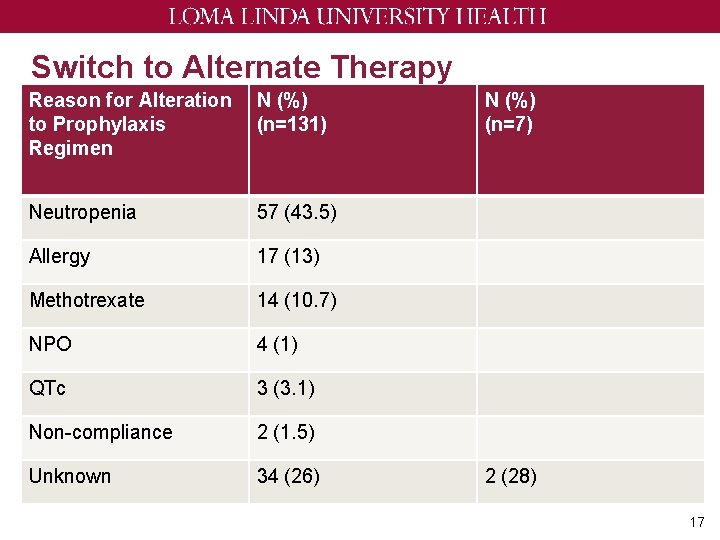
Switch to Alternate Therapy Reason for Alteration to Prophylaxis Regimen N (%) (n=131) Neutropenia 57 (43. 5) Allergy 17 (13) Methotrexate 14 (10. 7) NPO 4 (1) QTc 3 (3. 1) Non-compliance 2 (1. 5) Unknown 34 (26) N (%) (n=7) 2 (28) 17

Discussion ~ Only 1. 5% had confirmed PJP in twice weekly group • This is still higher than other studies ~ Not able to detect any PJP incidence among three times weekly due to low patient count ~ 15% of patients are switched to different prophylaxis regimen due to neutropenia ~ Most of these cases occur in the heme group • Longer duration of treatment • Treatments that cause neutropenia 18

Limitations • Retrospective ~ Bias ~ Unclear data points • Lack of control • Lack of follow-up • Small comparator group 19

Conclusion • Twice weekly dosing of Trimethoprim/Sulfamethoxazole had a similar incidence of PJP. • A larger multicenter study including local hospitals in is warranted to compare the efficacy of our institution’s practice to the common practice. 20

References 1. American Academy of Pediatrics. Pneumocystis jiroveci infections. In: Pickering LK, Baker CT, Long SS, et al. eds. 2015 2. Pneumocystis jirovecii Pneumonia Pediatric Opportunistic Infection. (2013, November 06) 3. Hughes WT, Rivera GK, Schell MJ, et al. Successful intermittent chemoprophylaxis for Pneumocystis carinii pneumonitis. N Engl J Med. 1987; 316: 1627– 1632. 4. Agrawal, Anurag K. , et al. “Twice Weekly Pneumocystis jiroveci Pneumonia Prophylaxis with Trimethoprim. Sulfamethoxazole in Pediatric Patients with Acute Lymphoblastic Leukemia. ” Journal of Pediatric Hematology/Oncology, vol. 33, no. 1, Jan. 2011. 5. Caselli, Désirée, et al. “Single-Day Trimethoprim/Sulfamethoxazole Prophylaxis for Pneumocystis Pneumonia in Children with Cancer. ” The Journal of Pediatrics, vol. 164, no. 2. 6. Lindemulder, S. , and E. Albano. “Successful Intermittent Prophylaxis with Trimethoprim/Sulfamethoxazole 2 Days per Week for Pneumocystis carinii (Jiroveci) Pneumonia in Pediatric Oncology Patients. ” Pediatrics, vol. 120, no. 1, Jan. 2007. 7. Ohata, Yasuhisa, et al. “Intermittent oral trimethoprim/Sulfamethoxazole on two non-Consecutive days per week is effective as Pneumocystis jiroveci pneumonia prophylaxis in pediatric patients receiving chemotherapy or hematopoietic stem cell transplantation. ” Pediatric Blood & Cancer, vol. 52, no. 1, 2009, pp. 142– 144. 8. Green, Hefziba, et al. “Prophylaxis of Pneumocystis Pneumonia in Immunocompromised Non-HIV-Infected Patients: Systematic Review and Meta-Analysis of Randomized Controlled Trials. ” Mayo Clinic Proceedings, vol. 82, no. 9, 2007, pp. 1052– 1059. , 9. Rossi, Mario R. , et al. “Prospective Randomized Comparison of Two Prophylactic Regimens with Trimethoprim -Sulfamethoxazole in Leukemic Children: a Two Year Study. ” European Journal of Cancer and Clinical Oncology, vol. 23, no. 11, 1987, pp. 1679– 1682. , 10. “Prevention and Treatment of Cancer-Related Infections. ” National Comprehensive Cancer Network, 1 Dec. 2017, 21

Thank you! » Dr. Caroline Sierra, Pharm. D, BCPPS » Dr. Monica Awad, Pharm. D » Dr. Khaled Bahjri, MD » Dr. Norm Hamada, Pharm. D » Steven La. Rue 22

Comparing two times weekly to three times weekly prophylaxis with Trimethoprim/Sulfamethoxazole for Pneumocystic jiroveci (carnii) pneumonia in pediatric oncology patients Elhaam Mesghali, Pharm. D. PGY-2 Pediatric Pharmacy Resident Loma Linda University Children’s Hospital | School of Pharmacy IRB Approved